Profiling of histone H3 trimethylation and distinct epigenetic pattern of chromosome Y in the transformed bronchial epithelial cells induced by consecutive arsenic treatment


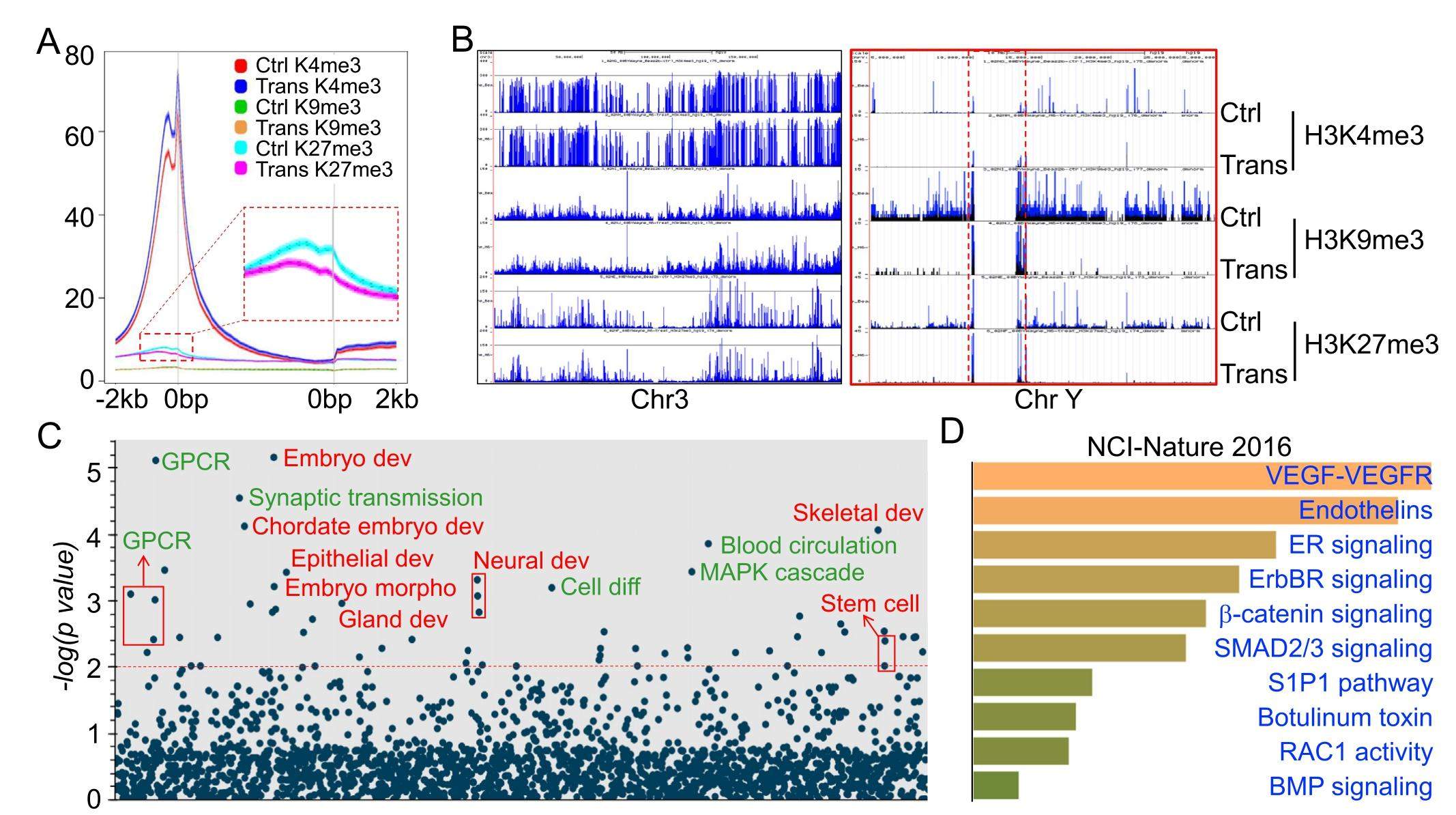
Environmental arsenic is a known human carcinogen that is associated with cancers in lung, liver, skin, breast, bladder, kidney, prostate, etc. However, the underpinnings of malignant transformation of the normal cells in response to environmental arsenic exposure remain poorly understood, in part due to mixed signaling pathways elicited by arsenic, especially the inorganic trivalent arsenic (As3+). In the present report we profiled the trimethylation status of histone H3 through global chromatin immunoprecipitation followed by sequencing (ChIP-seq) in the As3+-induced transformed cells and found an overall gain of active methylation of histone H3, the trimethylation of lysine 4 of histone H3 (H3K4me3), along with a diminished enrichment of the repressive marker H3K27me3 in the genome in the transformed cells relative to the control cells. Many oncogenic genes responsible for cell proliferation and selfrenewal of the cancer stem-like cells are enriched with H3K4me3 along with a reduced level of H3K27me3 and/or H3K9me3. In contrast, some genes encoding proteins with tumor suppressor-like activity showed reduced enrichment of the active marker, H3K4me3, but elevated levels of the repressive markers, H3K27me3 and H3K9me3. These data, thus, suggest that the effect of As3+ on histone methylations that define the chromatin configuration and gene expression dynamics of the genome and the cell fate decision may contribute significantly to the carcinogenicity of As3+.