Targeting DNA-PKcs promotes anti-tumoral immunity via triggering cytosolic DNA sensing and inducing an inflamed tumor immuno-microenvironment in metastatic triple-negative breast cancer


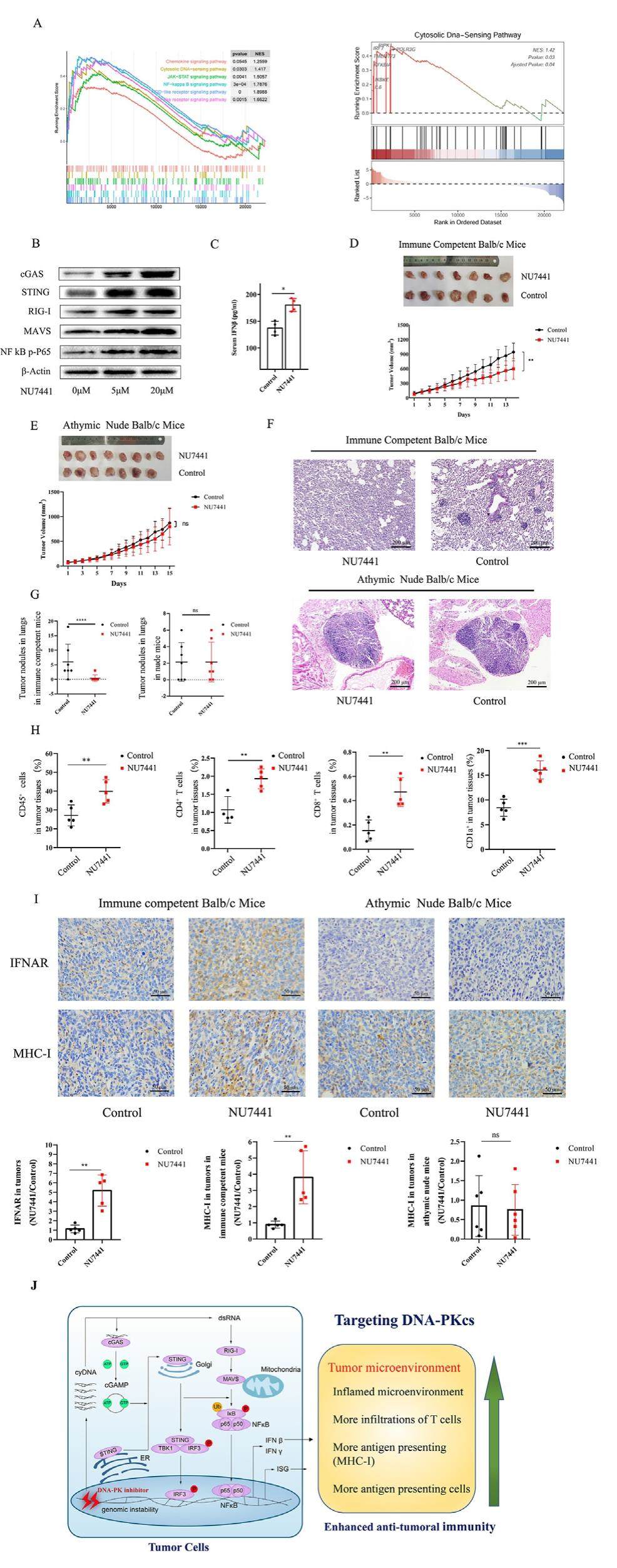
Triple-negative breast cancer (TNBC) is highly malignant and refractory to immunotherapy through impeding the immune cell infiltration and inflammation in the tumor microenvironment (TME). DNA-dependent protein kinase catalytic subunit (DNA-PKcs) is a member of the phosphatidylinositol 3-kinase-related kinase family, which is required for the nonhomologous end joining repair. The effect of DNA-PKcs on the generation of cytosolic DNA and inflammation response in tumor immuno-environment is not defined. We found a specific DNA-PKcs inhibitor, NU7441, induced cytosolic DNA, stimulator of interferon genes (STING), and retinoic acidinducible gene I (RIG-I) signals in vitro. In Balb/c immunecompetent mice bearing 4T1 TNBC cells, NU7441 impaired the tumor growth and metastasis, and increased the CD45+ leukocytes, CD4+ T cells, CD8+ T cells, and CD1a+ antigenpresenting cells, as well as MHC-I and interferon alpha receptor (IFNAR) inTME. However, inBalb/c athymic nudemice without IFNAR and CD8+ T cells in TME, NU7741 did not influence tumor growth. These results show that inhibition of DNA-PKcs triggers cytosolic DNA sensing and induces an inflamed TME to promote anti-tumoral immunity, which provides a strategy to alter the inflammation and lymphocyte infiltration in TME to increase the efficacy of immunotherapy in TNBC and other cancers with an immune-suppressive TME.