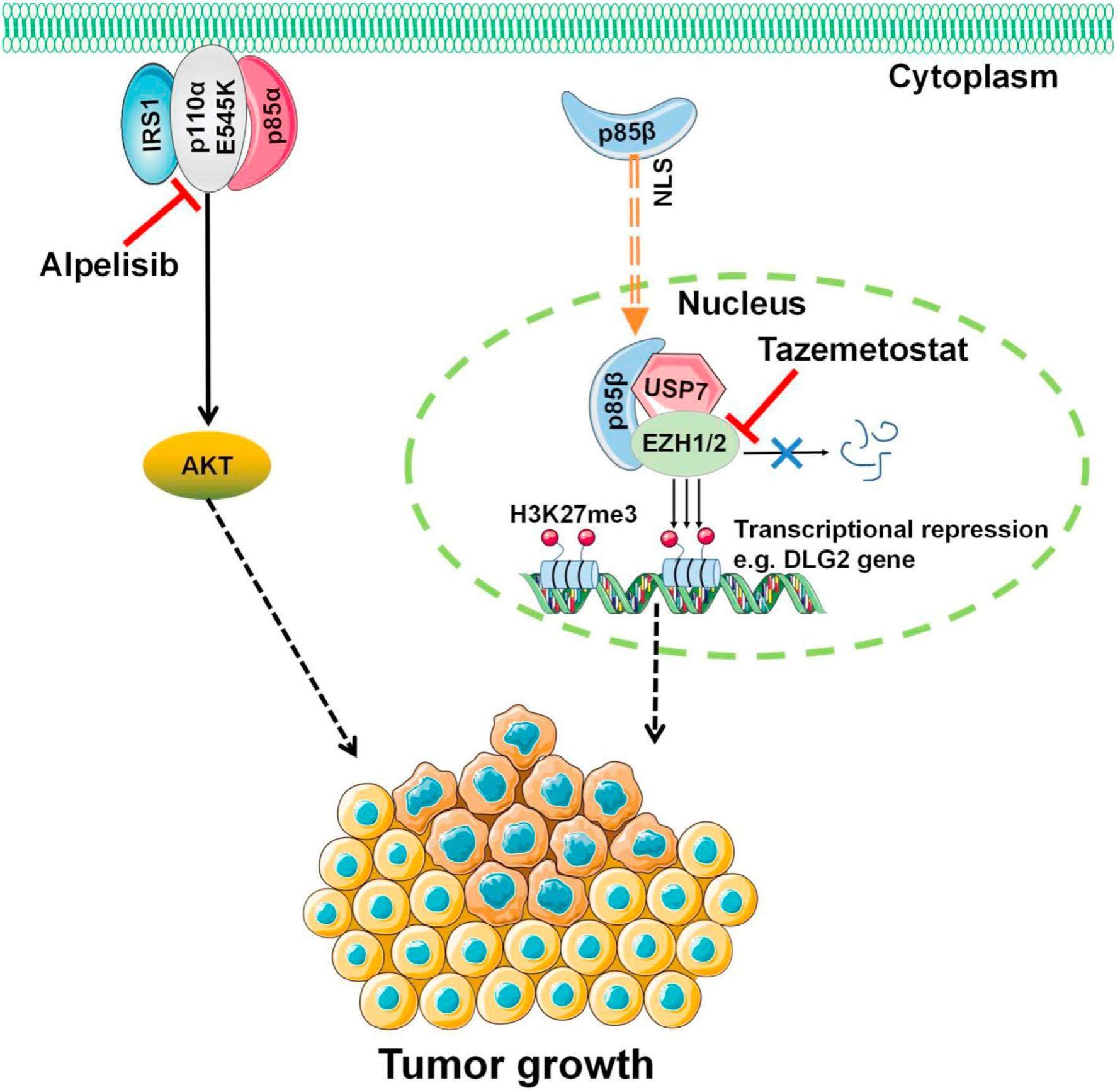
Targeting p85β nuclear translocation for the tumors with PIK3CA helical domain mutations


Phosphatidylinositol 3-kinases (PI3Ks) play key roles in tumorigenesis. PIK3CA, which encodes PI3K complex catalytic subunit p110α, is one of the most frequently mutated oncogenes in human cancers. So, targeting p110α holds great promise for cancer therapy. Alpelisib, a small molecule inhibitor specifically targeting PIK3CA/p110α, has been approved by FDA to treat HR-positive and HER2-negative breast cancer patients harboring PIK3CA gene mutations. Most PIK3CA/p110α mutations occur at two hot spot regions: an acidic cluster (E542, E545, and Q546) in the helical domain and a histidine residue (H1047) in the kinase domain. Although all these hot-spot mutations activate the PI3-kinase activity, p110α helical domain mutations and kinase domain mutations promote tumorigenesis through different molecular mechanisms. Moreover, PIK3CA helical domain mutant tumors were less responsive to alpelisib treatment compared with PIK3CA H1047R mutant tumors in early clinical trials, while the mechanism has not been clearly clarified. Therefore, it is important to investigate the oncogenic mechanism and develop a more effective therapeutic strategy for tumors with PIK3CA helical domain mutations.