Nuclear lncRNA CERNA1 enhances the cisplatin-induced cell apoptosis and overcomes chemoresistance via epigenetic activation of BCL2L10 in ovarian cancer


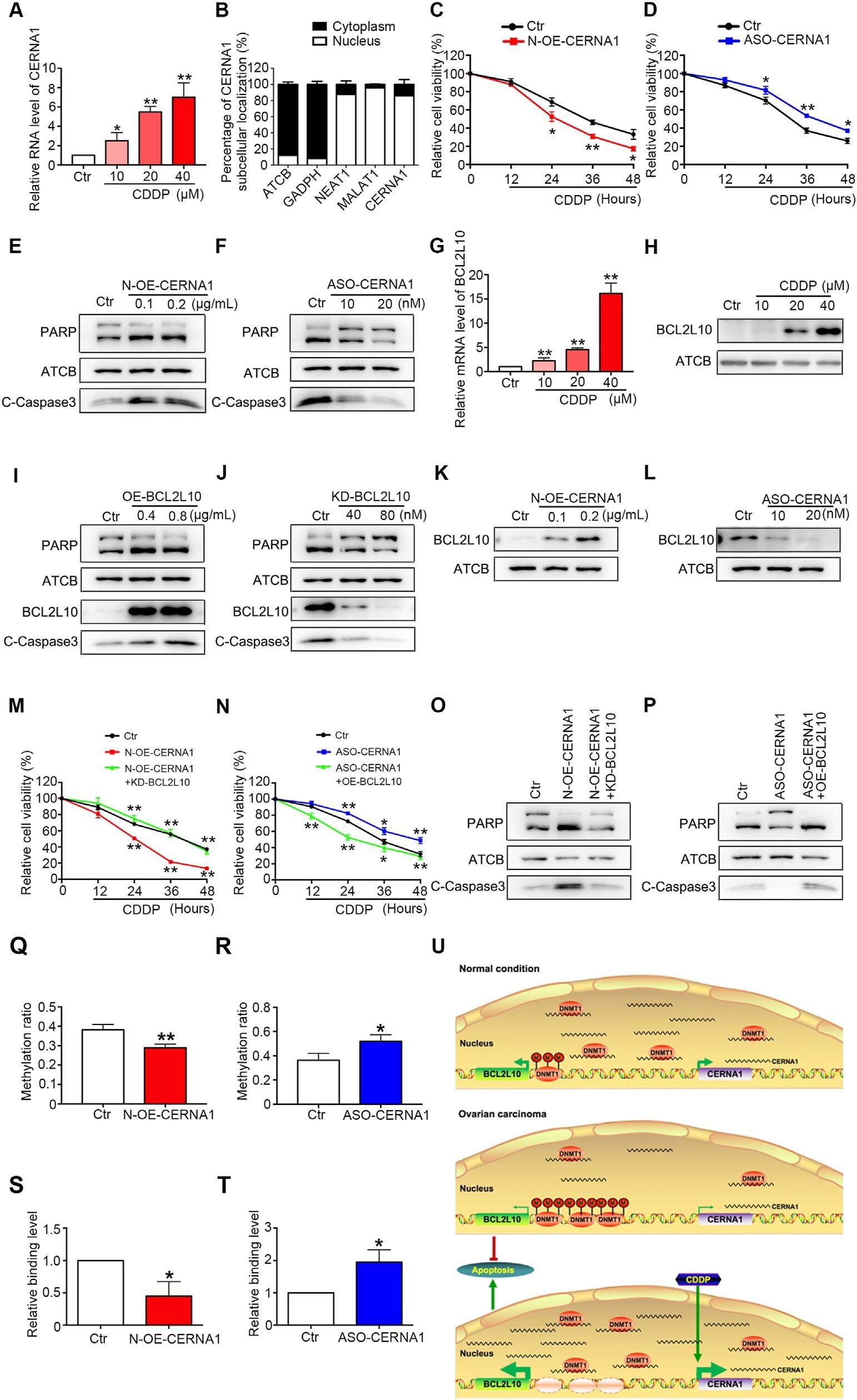
Ovarian cancer (OC) is the most lethal type of cancer among female genital tumors. Along with debulking surgeries, platinum is the first-line chemotherapy treatment, whereas chemoresistance is the biggest obstacles in a poor prognosis of OC. Identifying a novel therapeutic target for OC chemoresistance remains an urgent need. lncRNAs are expressed in tissue-specific patterns and distributed in the specific subcellular locations. The diverse regulatory mechanisms of lncRNAs are dependent on their subcellular location. lncRNA CERNA1, which is mainly located in the cytoplasm of HUVECs, acts as a miRNA sponge. Here, for the first time, we described the role and the molecular regulatory mechanism of nuclear CERNA1 in OC. We found that CERNA1 was poorly expressed in both OC tissues and OC cells, which predicts a poor OC prognosis. Unlike in HUVECs, CERNA1 is distributed almost exclusively in the nucleus of OC cells. Nuclear CERNA1 induced BCL2L10 expression (an OC tumor repressor), promoted cell apoptosis and reduced the cisplatin resistance by decoying DNMT1 from the BCL2L10 promoter. These results suggest that CERNA1 might be an attractive therapeutic target for improving the sensitivity to chemotherapy for OC patients.