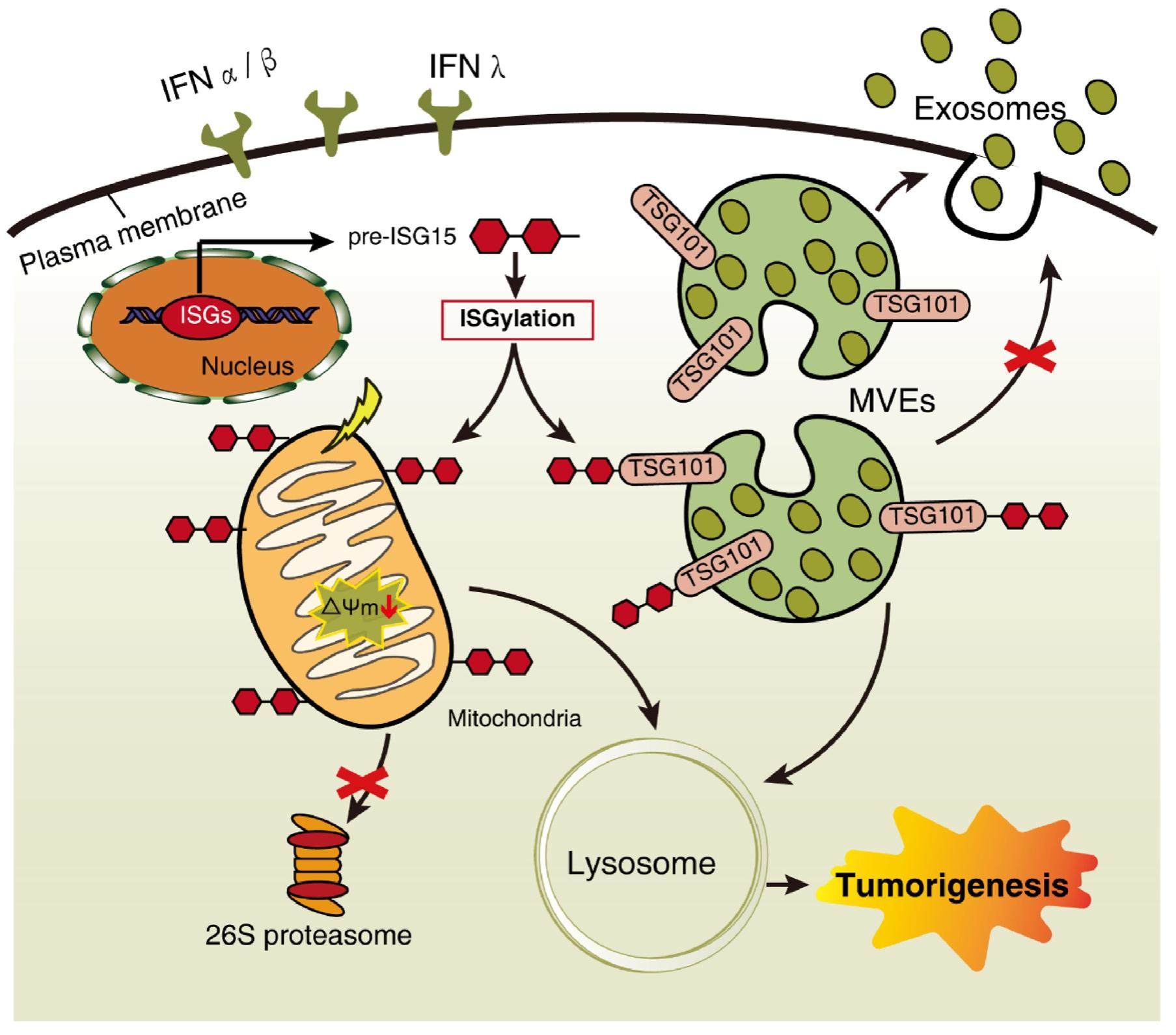
ISGylation orchestrates the degradation flux of cellular cargo toward lysosome


Post-translational modification (PTM) is a critical step for nascent protein processing, which accompanies with proteins lifetime to modulate their functions dynamically. Currently, there are over 300 species of PTMs been identified, including chemical group modification (e.g., phosphate, acetyl, methyl, or succinyl groups) and ubiquitinlike (UBL) group modification (e.g., ubiquitination, SUMOylation, and ISGylation). The UBL modification covalently linked to the side chain of amino acid residue, like lysine, that signal for multiple biological progresses. Ubiquitin is firstly identified as a modification on protein, that involved in a variety of metabolic processes, such as mitophagy. ISGylation, like is a unique PTM among UBL conjugation that known to be induced by specific inflammatory stimuli, such as interferon signaling pathway, its novel function on mediating mitophagy and exosome secretion was recently uncovered, in particular on tumorigenesis.