Menin facilitates the cell proliferation of bladder cancer via modulating the TFAP2C/β-catenin axis


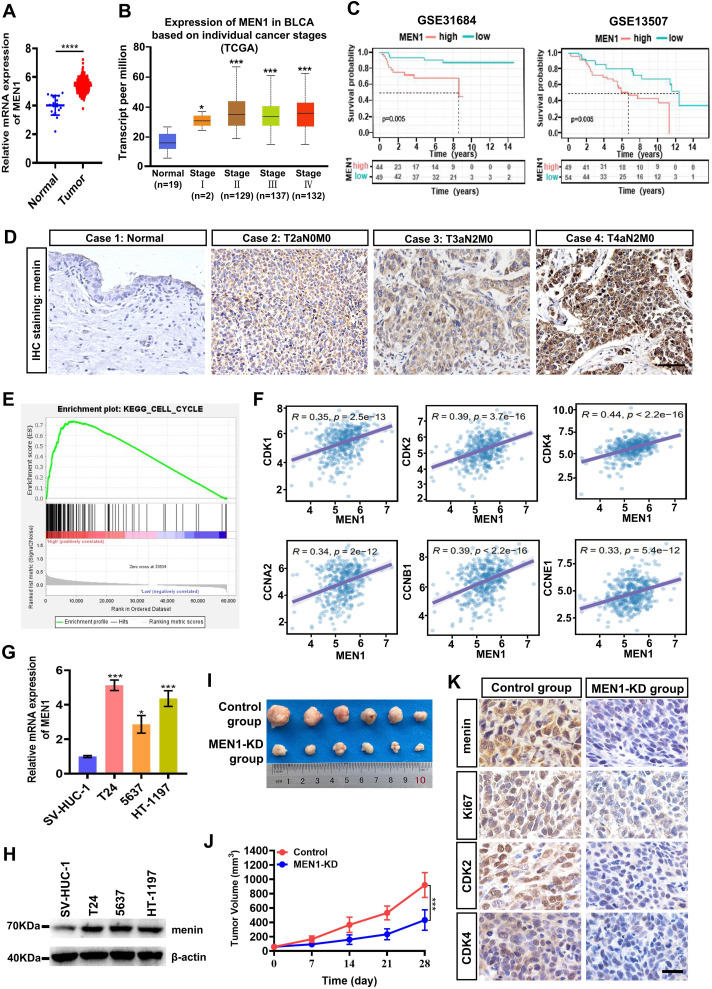
Bladder cancer (BLCA) is a common malignant tumor of the urinary system, with significant morbidity and mortality rates worldwide. The MEN1 gene, encoding the menin protein, plays a regulatory role in several cancers. However, the role played by menin in BLCA remains elusive. In this study, our data demonstrated that the expression of menin was significantly up-regulated in BLCA tissues versus normal tissues, and the high expression of menin was strongly correlated with poor prognosis of BLCA patients. In vitro, silencing MEN1 inhibited cell proliferation and induced cell cycle arrest at the G1/S phase in BLCA cells. Furthermore, RNA sequencing analysis revealed that MEN1 knockdown significantly inhibited the Wnt/β-catenin signaling in BLCA cells. Meanwhile, we further confirmed that β-catenin served as a critical downstream effector of menin in BLCA cells. Mechanically, chromatin immunoprecipitation analysis demonstrated that menin promoted CTNNB1 (catenin beta 1) transcription through binding to the CTNNB1 proximal promoter in BLCA cells. Interestingly, menin collaborated with TFAP2C, a regulator of β-catenin in BLCA cells, to enhance the transcription of the CTNNB1 gene. More intriguingly, BAY-155, a menin molecule inhibitor, inhibited cell growth of BLCA cells both in vitro and in vivo by suppressing the expression of menin, TFAP2C, and β-catenin. Our current work unveils an important role of the menin in triggering the TFAP2C/β-catenin axis, which contributes to cell proliferation of BLCA cells. Therefore, menin might be served as a new therapeutic target for BLCA.