A simplified noncryogenic strategy to transport mesenchymal stem cells: Potential applications in cell therapy and regenerative medicine


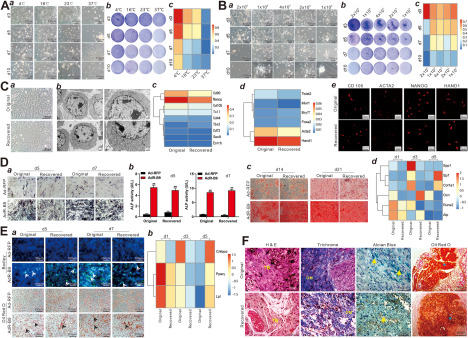
With the rapid advances in stem cell research and potential cell-based therapies, there is an urgent need to develop safe and reliable cell transport strategies. Except for autologous stem cell-based therapies, allogeneic stem cell therapies and ex vivo genetically engineered cell therapies would require safe, efficient, and reliable cell preservation and transport methods.1 Conventional cell transport methods include shipping live cells in medium-filled culture flasks at room temperature or ambient/hypothermic conditions (<35 °C) within several days, or expeditiously transporting cryogenically preserved cell vials in dry ice or liquid nitrogen,2 as it is commonly believed that hypothermic preservation benefits cell survival by reducing cellular metabolic rate and oxygen demand.3 While the former approach is convenient with variable outcomes, the latter is expensive, environmentally unfriendly, and technically challenging for long-distance transport. Here, using the mesenchymal stem cell (MSC) iMEFs as a model cell line,4 we developed a simplified, inexpensive, and noncryogenic method to transport cells, especially. By optimizing several crucial parameters including storage temperature, cell density, fetal bovine serum (FBS) concentration, and pH stability, we found that the cell viability remained 16%–18% when 2–10 × 105 cells were stored in 1 mL of 2% FBS DMEM in a 1.5 mL Eppendorf tube at 4 °C–16 °C for up to 10 days. Furthermore, we demonstrated that the MSCs recovered from the above conditions maintained normal morphology, expressed stem cell markers, and retained osteogenic and adipogenic differentiation potential upon BMP9 stimulation in vitro and in vivo. Thus, our study identified an inexpensive, noncryogenic and reliable strategy for storage and transport of MSCs, potentially most mammalian cells.