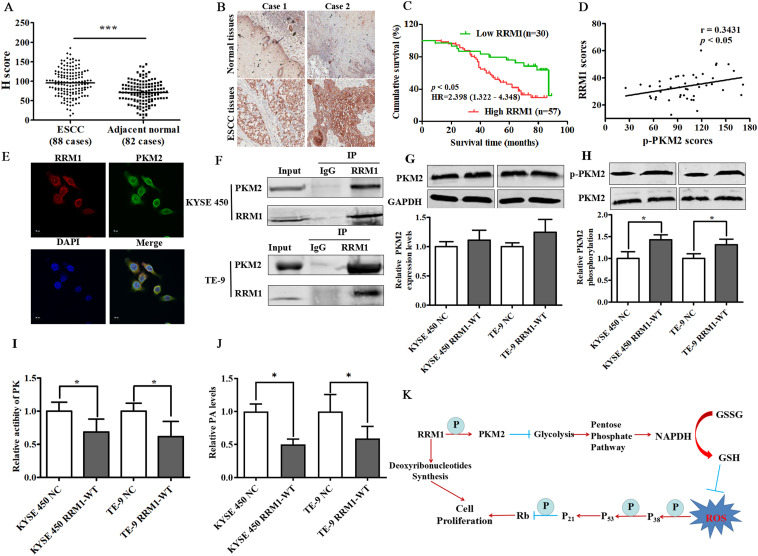
Ribonucleotide reductase subunit M1 blocks glucose catabolism via phosphorylation of pyruvate kinase M2 in human esophageal squamous cell cancer


Esophageal squamous cell cancer (ESCC) is one of the malignant tumors with high morbidity and mortality all over the world.1 In recent years, combined chemotherapy has gradually been the effective treatment method for ESCC patients. Therefore, it is imperative to explore potential therapeutic targets for the treatment of ESCC. We previously reported a high-frequency amplification of pyrimidine metabolic pathway-related genes in ESCC tissues,2 and ribonucleotide reductase subunit M1 (RRM1) was one of many abnormal genes. RRM1 is one of the important subunits of ribonucleoside reductase (RR) and plays a key role in cell DNA replication as a binding site of nucleotides to regulate substrate specificity and RR activity. RRM1 is expressed in almost all tumor cells, but its expression levels and functions are differently affected by tissue origin and cell location. The expression of RRM1 in lung cancer tissues was generally lower than that in adjacent tissues, while an opposite trend was observed in breast cancer. Reports about RRM1 in ESCC are less, and the mechanism(s) of carcinogenesis is still poorly understood. The present study aimed to understand the role of RRM1 on the malignant proliferation process, and further clarify the molecular mechanism in ESCC development, which would be helpful in anti-cancer therapy of ESCC patients.