Cysteine mutations impair the structural stability of phosphomannomutase 2 (PMM2) in glycosylation-associated metabolic disorders


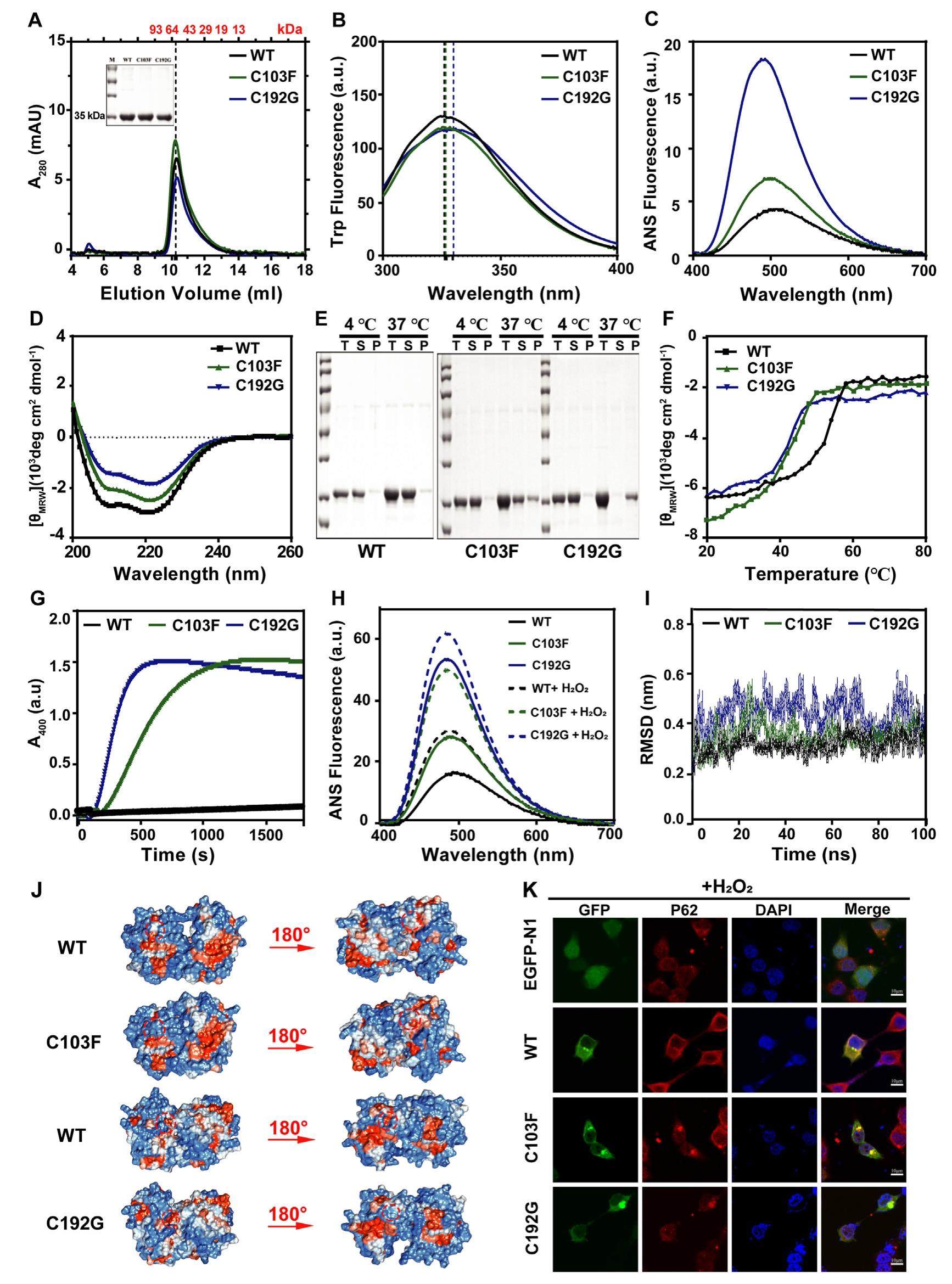
Glycosylation is a ubiquitous molecular modification in the process of cellular metabolism regulation, which plays a vital role in maintaining and regulating cellular functions. Deficiency in glycosylation enzymes may jeopardize the glycosylation process and eventually lead to the metabolic disease, manifesting with either a neurologic or multisystem phenotype, which is also known as congenital disorder of glycosylation (CDG). It could be classified into type I CDG and type II CDG, and the latter was referred to as the abnormal formation of nascent glycoprotein. Phosphomannomutase 2 CDG (PMM2-CDG), the most prevalent CDG with an incidence rate of 1 in 20, 000 individuals, is mainly due to the metabolic disorder of phosphomannomutase 2 (PMM2). PMM2 participates in the conversion of mannose-6-phosphatetomannose-1-phosphate. The cysteine residues, as one of the least abundant amino acids, have unique attributes to maintain the stability of protein structure, especially for catalytic activity and protein folding. Therefore, cysteine mutations have been identified in various diseases including PMM2-CDG. Suggested by the crystal structure of PMM2 (PDB ID: 7O5Z), four cysteine mutations in the hPMM2, C9Y, C103F, C192G, and C241S, have been found in PMM2-CDG. After bioinformatic analysis by PROVEAN, the four cysteine mutations were predicted with "Deleterious", ranking with the best two were C103F and C192G (C9Y: -5.493, C103F: -6.339, C192G: -11.176, C241S: -2.986). However, the underlying pathological mechanism is still unclear. Among them, C103F and C192G have only been reported in clinical study without a detailed explanation, so they were selected for our research. Two mutants were located near the linker region 1 and 2; C103F was located on the No. 4 a helix and C192G was located on the No. 7 a helix. The purpose of this study was to explore the structure and function roles of PMM2 clinical mutation and investigate the role of cysteine in PMM2 which might provide new insights into preventive and therapeutic strategies for PMM2-CDG.