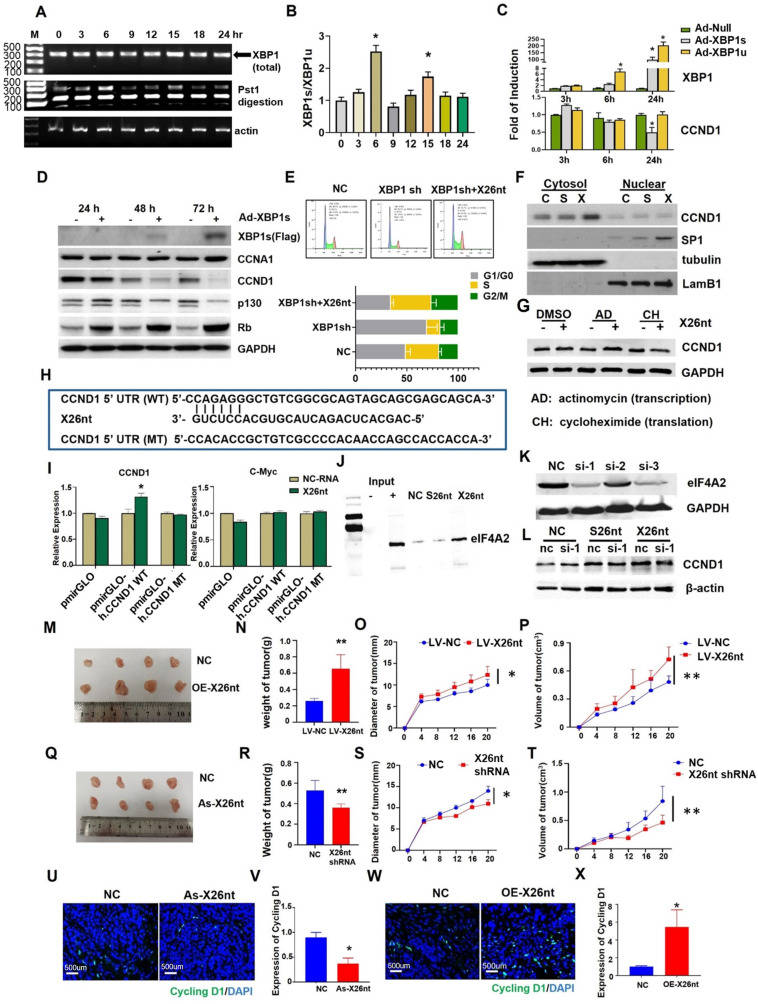
X26nt-mediated recruitment of eIF4A2 facilitates CCND1 translation to drive endothelial cell cycle progression


Dysregulation of cell cycle control plays a pivotal role in tumor progression. Acting as a crucial regulator of the G1/S phase transition, dysregulation of cyclin D1 (CCND1) can disrupt the delicate balance between cell proliferation and quiescence, leading to uncontrolled cell cycle progression.1 Endoplasmic reticulum stress influences the cell cycle through myriad pathways. During endoplasmic reticulum stress, the inositol-requiring enzyme 1 alpha (IRE1α) can be activated, and its endoribonuclease domain cleaves unspliced X-box binding protein 1 (XBP1u) mRNA, generating a spliced XBP1 (XBP1s) and a non-coding RNA, whose length is 26 nt (named exosomal 26-nt-long ncRNA, X26nt).2,3 Studies have demonstrated that XBP1s can induce the expression of pro-angiogenic factors, promoting the formation of new blood vessels.4,5 Our previous study demonstrated that gastric cancer exosome-derived X26nt induces tumor-associated angiogenesis via promoting endothelial cell migration targeting vascular endothelial-cadherin.2 However, the exact mechanisms by which XBP1 splicing regulates endothelial cell proliferation are still not fully understood.