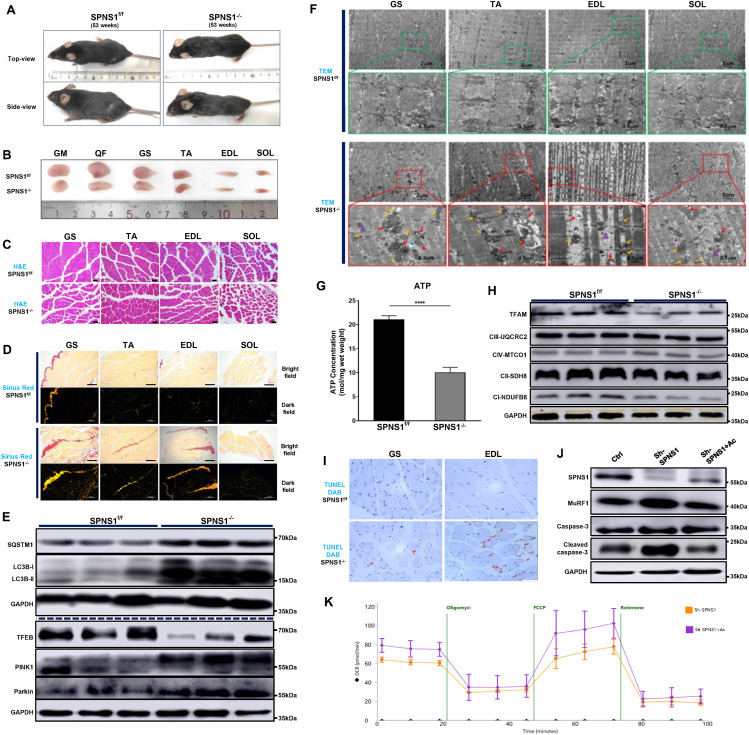
SPNS1 ablation drives skeletal muscle atrophy by disrupting mitophagy, mitochondrial function, and apoptosis in mice


Sarcopenia is characterized by a dramatic decline in muscle mass and function during aging, which often leads to reduced mobility, diminished quality of life, and shortened survival. Despite recent advances in sarcopenia, the mechanisms that mediate skeletal muscle dysfunction remain unclear.1 Lysosomal plays a vital role in digesting and recycling macromolecules, and several studies have shown that lysosomal acidification and autophagy decrease with age, which may impair the clearance of damaged mitochondria.2 SPNS1 (spinster homolog 1), a proton-dependent lysophosphatidylcholine and lysophosphatidylethanolamine transporter, plays a critical role in maintaining normal lysosomal function.3 However, the physiological relevance of lysosomal dysfunction in sarcopenia remains unclear. Coffey et al found that loss of SPNS1 dysregulated lysosomal pH, which impacts the expression of extracellular matrix proteins at the myotendinous junction in zebrafish.4 In this study, we demonstrate that ablation of SPNS1 in skeletal muscle results in impaired lysosomal digestion, hindering mitophagy and triggering the accumulation of abnormal mitochondria. As a result, damaged mitochondria cannot be cleared, leading to the leakage of pro-apoptotic protein and the activation of apoptosis. Our findings reveal the role of SPNS1 in maintaining mitochondrial-lysosomal homeostasis and preserving muscle mass and function.