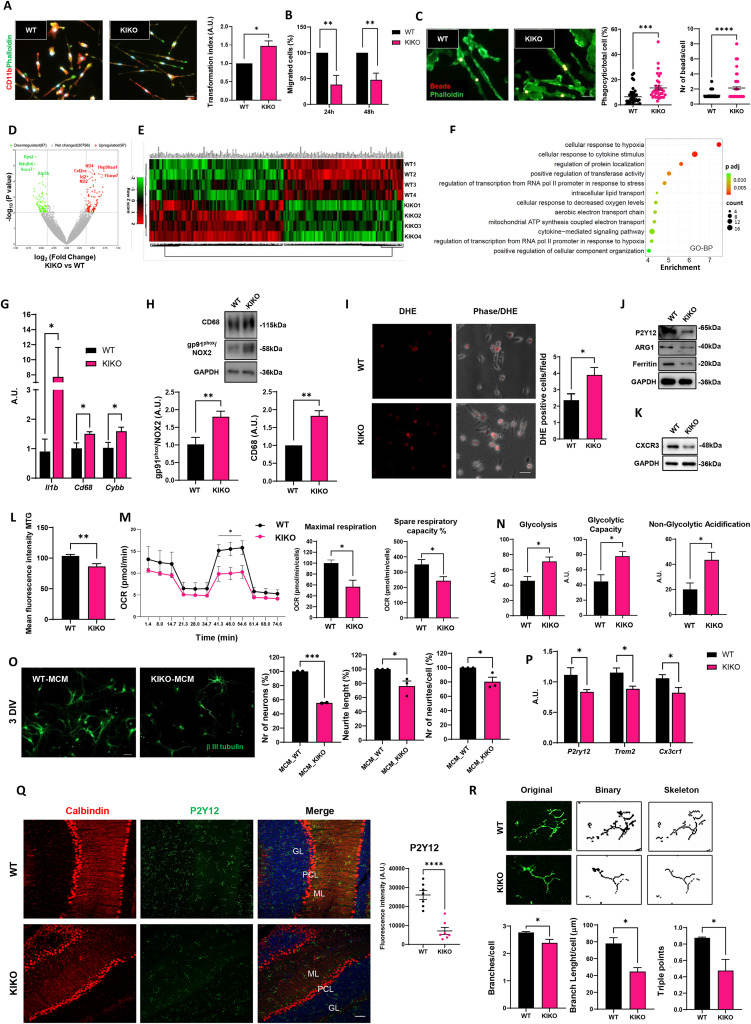
Loss of homeostatic functions in microglia from a murine model of Friedreich's ataxia


Friedreich's ataxia (FRDA) is a rare genetic disorder characterized by motor discoordination and cerebellar involvement due to mutations in the frataxin (FXN) gene, which encodes a mitochondrial protein involved in iron-sulfur cluster biogenesis and iron handling.1 While progress has been made in understanding FRDA's pathophysiology and cerebellar degeneration caused by frataxin deficiency, the role of central nervous system (CNS)-resident non-neuronal cells, as microglia, necessitates further investigation. Microglia play crucial roles in CNS development, neurogenesis, apoptosis, and synaptic remodeling, acting as sentinels with homeostatic functions. In neurodegenerative diseases, microglia respond rapidly to injury, potentially leading to sustained neuroinflammation and therefore, contributing to foster neuron damage.2 Similarly, in FRDA, cerebellar neuron degeneration may be influenced by glial cells in a non-cell-autonomous process. Indeed, in FRDA, microglia contribute to reactive oxygen species accumulation in the CNS, particularly in cerebellar regions, possibly participating in cerebellar susceptibility in ataxias. Circumstantial evidence supports the involvement of neuroinflammatory mechanisms in FRDA pathogenesis,3 as indicated by the presence of hypertrophic and reactive microglia in brain regions of FRDA mouse models and patients, with increased neuroimmune activity correlating with earlier symptom onset and shorter disease duration.4 However, the extent to which microglia dysfunction contributes to FRDA remains uncertain.