Prevalence of synonymous mutations in m6A modification sites in human cancers


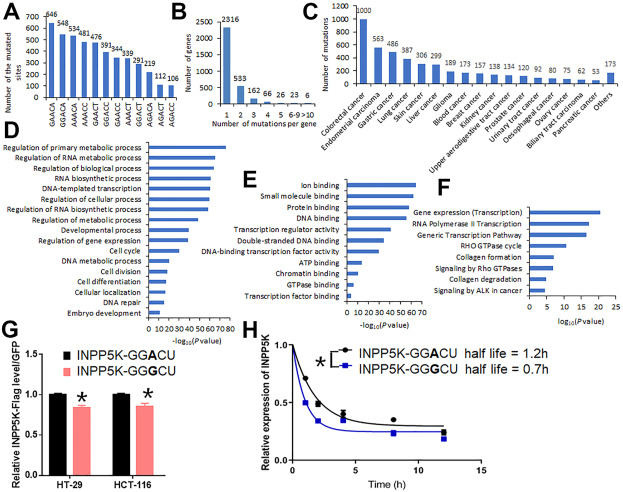
As one amino acid can often be encoded by multiple codons, the genetic code is redundant, which accounts for synonymous mutations in protein-coding regions.1 Since synonymous mutations do not cause any alterations in amino acid sequence, it was previously believed that they do not change the structure and function of the proteins and thus are functionally silent.1 However, evidence is emerging that synonymous mutations can affect messenger RNA (mRNA) stability, splicing and translation, and thereby influencing protein biogenesis.1 Nevertheless, the detailed mechanisms underlying the impacts of synonymous mutations on mRNA metabolism and protein biogenesis remain elusive. N6-methyladenosine (m6A), the most prevalent internal modification in eukaryotic mRNA, has been shown to play important roles in gene regulation in both normal and disease cells, through post-transcriptional regulation of mRNA stability, splicing and translation.2 Therefore, we assumed that if synonymous mutations occur in RNA m6A modification sites, synonymous mutations could change mRNA m6A modifications, which in turn would affect mRNA stability, splicing and translation. Indeed, through overlapping synonymous mutations in the classic m6A motif DRACH (D = A, G or U; R = G/A; H = A, C or U) sites in human cancers listed in the COSMIC (the Catalogue of Somatic Mutations in Cancer) database (https://cancer.sanger.ac.uk)3 with the detected m6A peaks listed in the RMBase database,4 we found that around 4500 synonymous mutations in over 3000 genes occurred at the “A” site of the classic m6A motifs DRACH with detected m6A peaks. Thus, our data reveals the prevalence of overlapping between synonymous mutations and m6A sites in genes in human cancers, suggesting that synonymous mutations-mediated m6A modification changes may contribute to the changes in the expression of the target genes in human cancers.