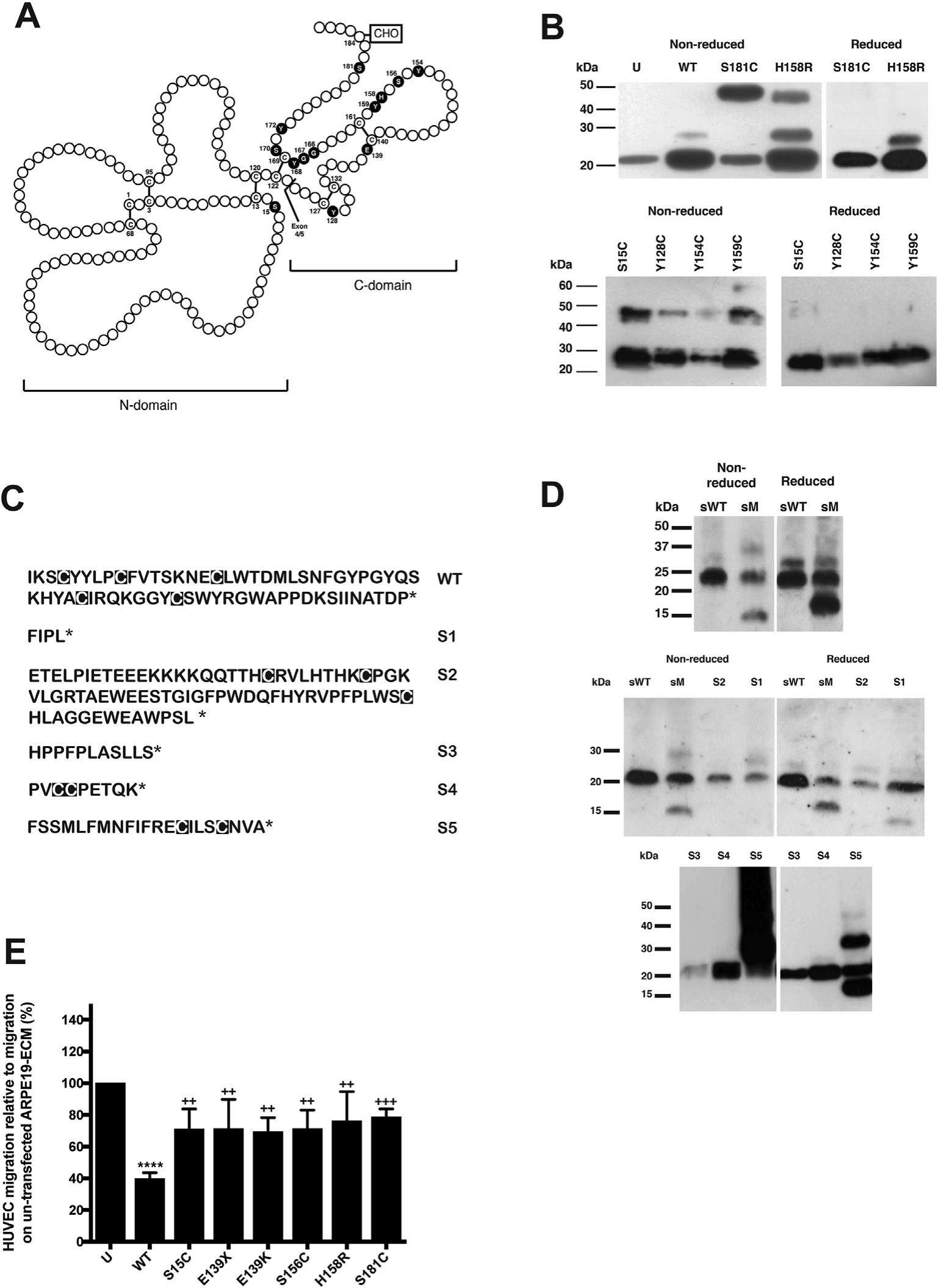
Evidence that all Sorsby's fundus dystrophy mutations cause TIMP3 dimerization resulting in impaired inhibition of VEGFR2


Tissue inhibitor of metalloproteinases 3 (TIMP3) regulates turnover of the extracellular matrix (ECM) and is also a potent inhibitor of the vascular endothelial growth factor receptor 2 (VEGFR2), a key mediator of angiogenesis. Mutations in TIMP3 give rise to Sorsby's fundus dystrophy (SFD), a dominantly inherited degenerative disease of the retina that leads to blindness, usually in middle age. To date, fifteen different mutations in TIMP3 have been identified as causing the disease. Conventionally, these were numbered for their positions in the secreted protein, excluding the signal sequence but are referenced in Table S1, with and without including the signal sequence; however, we use the conventional nomenclature in the text. illustrates their relationship to the primary structure. Thirteen of these are missense mutations, one a nonsense mutation and one a mutation in the splice acceptor site at the intron 4/exon 5 boundary. All but one of these mutations (S15C) affect exon 5, which codes for all except the first 3 residues of the carboxyl-terminal domain of the molecule. Notably, eleven mutations result in a change to a cysteine residue, while the nonsense mutation (E139X), also gives rise to an unpaired cysteine residue. It seems unlikely that this is coincidental. Indeed, we have shown many of these Cys residues result in dimerization and/or multimerization of the TIMP3 protein. Moreover, the E139K missense mutation and the E139X nonsense mutation have also been shown to form disulfide bonded dimers, strengthening the hypothesis that TIMP-3 dimerization is a prerequisite for the disease. However, the H158R mutation and the S15C mutation have both been reported to be solely monomeric, challenging this hypothesis. Moreover, the molecular consequences of several other mutations, and the novel splice acceptor site mutation have never been examined.