Single-cell transcriptomics illustrates the immune inflammatory responses of septic mice spleen after capsaicin treatment


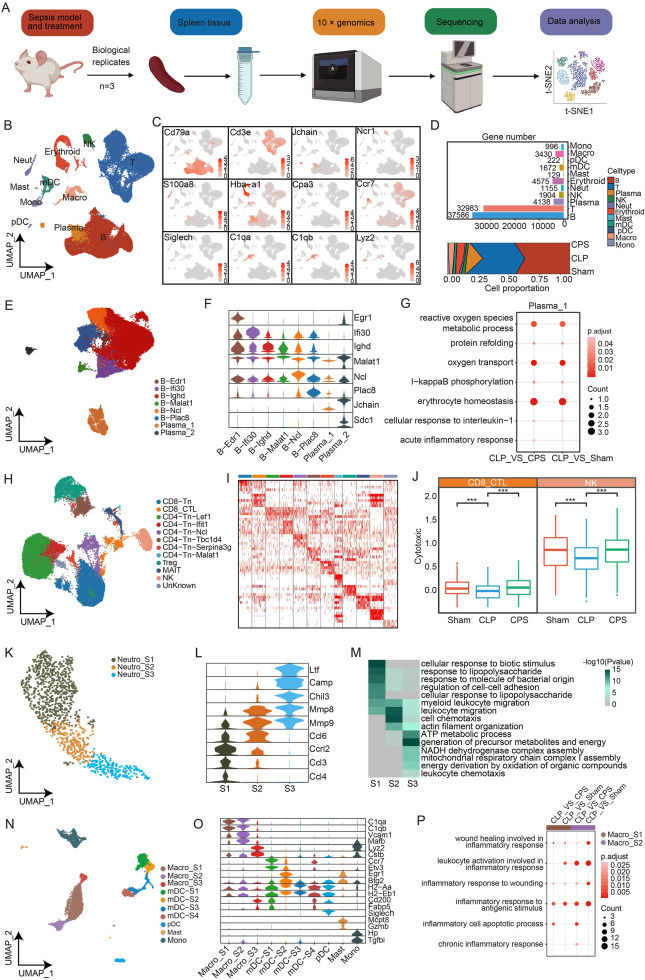
Sepsis, a life-threatening condition triggered by a dysregulated host response to infection, remains a major challenge for therapeutic intervention. Despite the growing interest in immunomodulatory strategies for sepsis treatment, the effects and mechanisms of these approaches on the organ-specific inflammatory and immunosuppressive states induced by sepsis are poorly understood. According to existing studies, capsaicin (CPS) has pharmacological effects such as analgesic, antipruritic, hypolipidemia, hypoglycemia, anti-inflammatory and antibacterial, and anti-tumor activity.1 However, the effectiveness of CPS in treating sepsis is still unknown. Here, we applied single-cell RNA sequencing (scRNA-seq) to reveal how CPS, a natural compound with anti-inflammatory properties, modulates the splenic microenvironment in a mouse model for sepsis induced by cecal ligation and puncture (CLP). We found that CPS improved survival and reduced inflammation in septic mice. Additionally, scRNA-seq analysis revealed that CPS plays an antioxidant and anti-inflammatory role in the body by regulating plasma cells derived from B cells. Moreover, CPS activated cytotoxic T cells and natural killer cells. Furthermore, we have observed that CPS has the potential to regulate cytokine production but exerts limited influence on the chemotactic activity of neutrophils during sepsis. Lastly, CPS enhanced the polarization of inflammatory macrophages during sepsis. Hence, our findings imply that CPS holds the potential to harmonize immune homeostasis, thereby dampening the inflammatory and/or immunosuppressive milieu observed in septic conditions. Our investigation presents a comprehensive scrutiny of CPS's role in orchestrating the spleen microenvironment in the context of septic infection, offering a foundational rationale for considering CPS as a prospective therapeutic intervention against sepsis.