Nucleolus localization of SpyCas9 affects its stability and interferes with host protein translation in mammalian cells


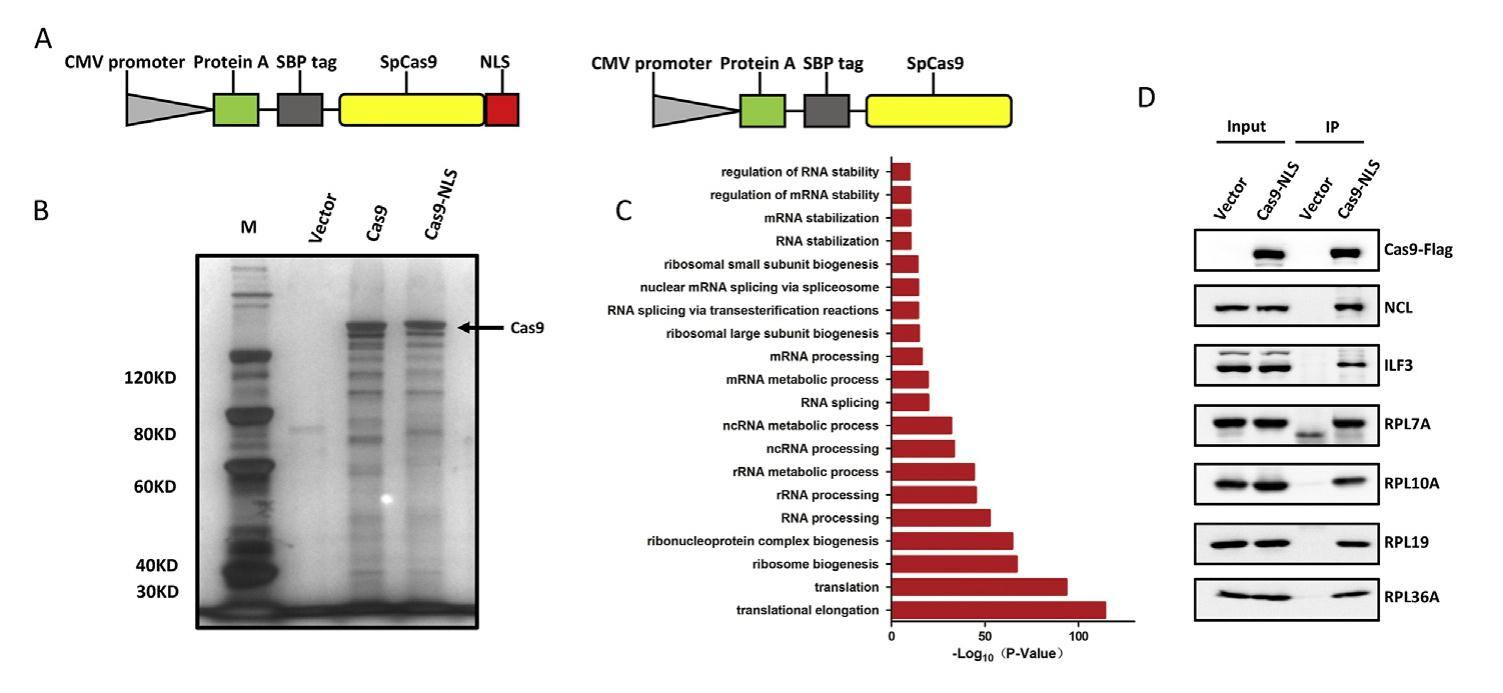
The CRISPR/Cas9 system, originally derived from the prokaryotic adaptive immune system, has been developed as efficient genome editing tools. It enables precise gene manipulation on chromosomal DNA through the specific binding of programmable sgRNA to target DNA, and the Cas9 protein, which has endonuclease activity, will cut a double strand break at specific locus. However, Cas9 is a foreign protein in mammalian cells, and the potential risks associated with its introduction into mammalian cells are not fully understood. In this study, we performed pull-down and mass spectrometry (MS) analysis of Streptococcus pyogenes Cas9 (SpyCas9) interacting proteins in HEK293T cells and showed that the majority of Cas9-associated proteins identified by MS were localized in the nucleolus. Interestingly, we further discovered that the Cas9 protein contains a sequence encoding a nucleolus detention signal (NoDS). Compared with wild-type (WT) Cas9, NoDS-mutated variants of Cas9 (mCas9) are less stable, although their gene editing activity is minimally affected. Overexpression of WT Cas9, but not mCas9, causes general effects on transcription and protein translation in the host cell. Overall, identification of NoDS in Cas9 will improve the understanding of Cas9's biological function in vivo, and the removal of NoDS in Cas9 may enhance its safety for future clinical use.