Lipid catabolism and mitochondrial uncoupling are stimulated in brown adipose tissue of amyotrophic lateral sclerosis mouse models


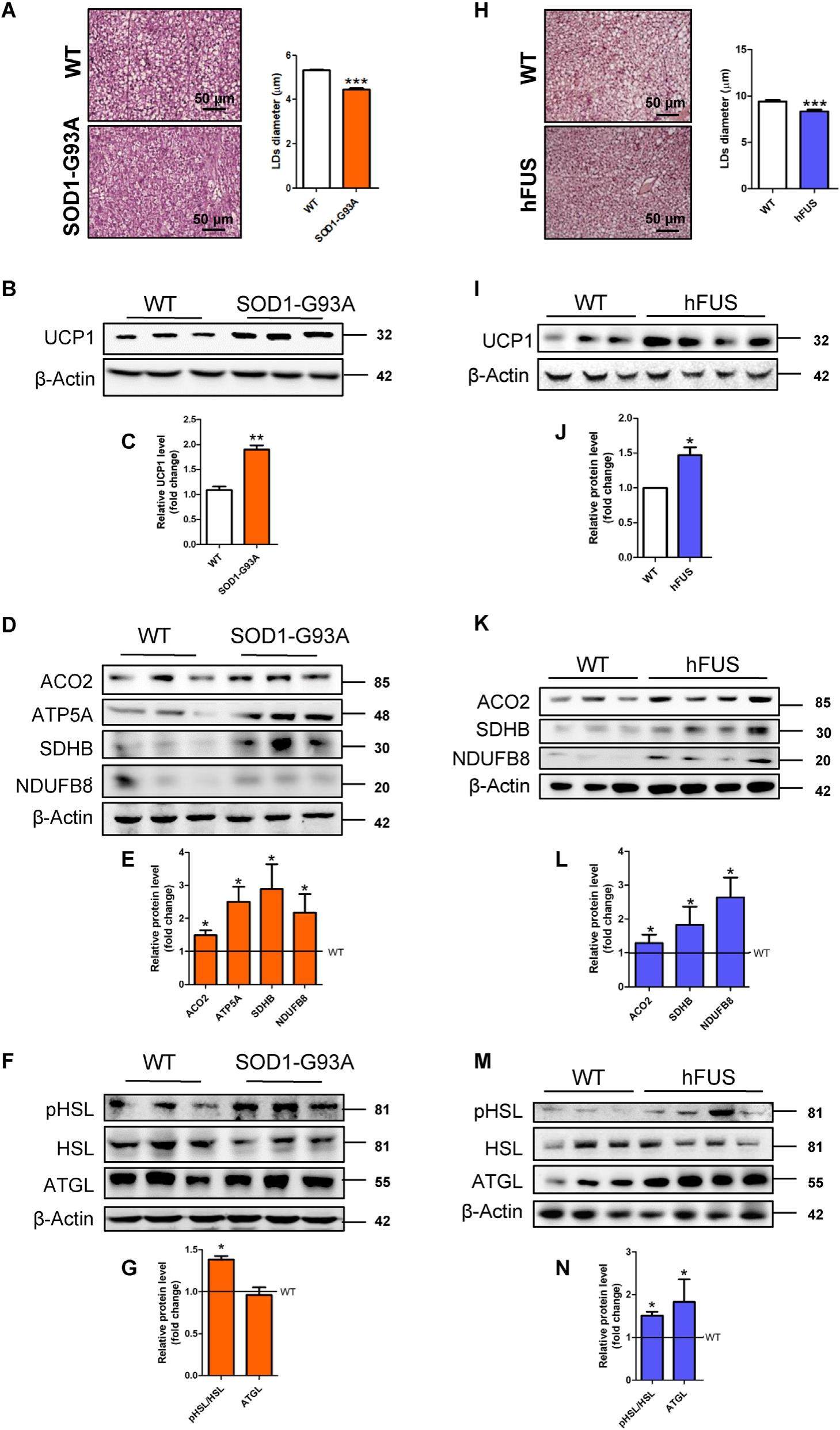
Amyotrophic lateral sclerosis (ALS) is a fatal motor neuron disease typically leading to death within 5 years from symptom onset. ALS familial forms are associated with mutations in several genes, including Superoxide Dismutase 1 (SOD1) and Fused in Sarcoma (FUS). Although genes linked to ALS participate in disparate biological processes, ALS genetic variants largely trigger shared pathogenic events such as oxidative stress, protein aggregation and defects in RNA processing. Moreover, ALS patients show systemic hypermetabolism that leads to increased energy expenditure at rest and thus weight loss during the disease course. ALS hypermetabolic phenotype and weight loss have been extensively characterized in mice bearing the G93A substitution in SOD1 protein (SOD1-G93A), which exhibit skeletal-muscle metabolic reprogramming before disease onset.