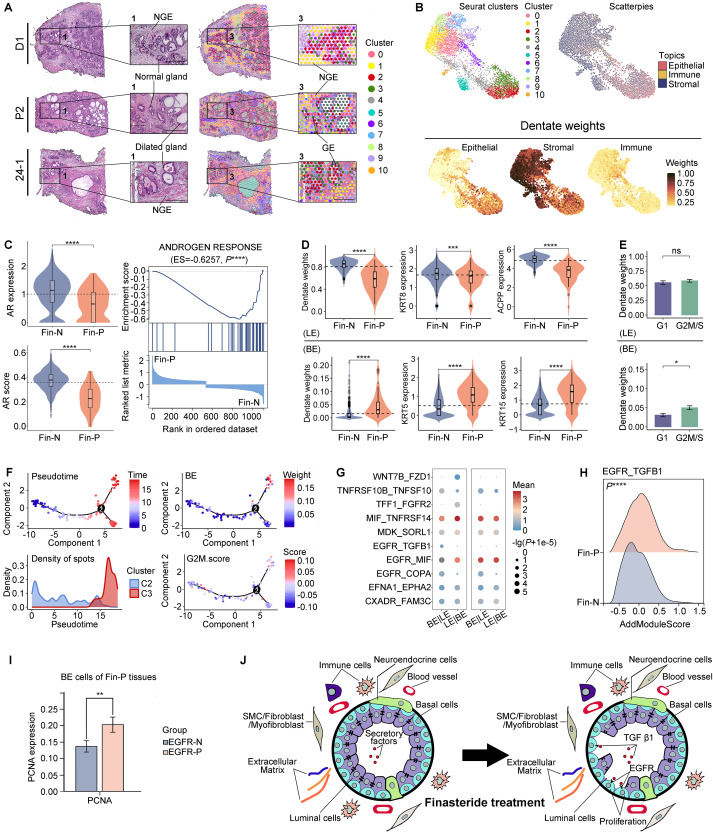
Integrating spatial transcriptomics and single-cell RNA-sequencing reveals epithelial cell alterations in benign prostatic hyperplasia following finasteride treatment


The pathogenesis of benign prostatic hyperplasia (BPH) is commonly regarded as androgen-dependent. The first FDA-approved androgen-targeted medication for BPH, finasteride, achieves its therapeutic effect by selectively inhibiting type II 5-alpha reductase (SRD5A2). This inhibition reduces the androgenic response by attenuating the interaction between dihydrotestosterone and androgen receptor (AR), ultimately leading to a reduction in prostate volume and alleviation of BPH-associated symptoms. However, it is noteworthy that non-response to finasteride may develop in certain BPH patients,1 indicating a potential inadvertent promotion of disease progression by this treatment. Nevertheless, the underlying mechanism remains elusive. Recent researches suggest that it may be associated with alterations within the prostate gland epithelia.2 Therefore, we designed this study to elucidate this phenomenon by employing spatial transcriptomic (ST) and single-cell RNA sequencing (scRNA-seq) (methods were described in Supplementary Materials in detail). Our findings will improve treatment adjustments by enabling a more accurate evaluation of patient's response to finasteride therapy, thus enhancing therapeutic outcomes.