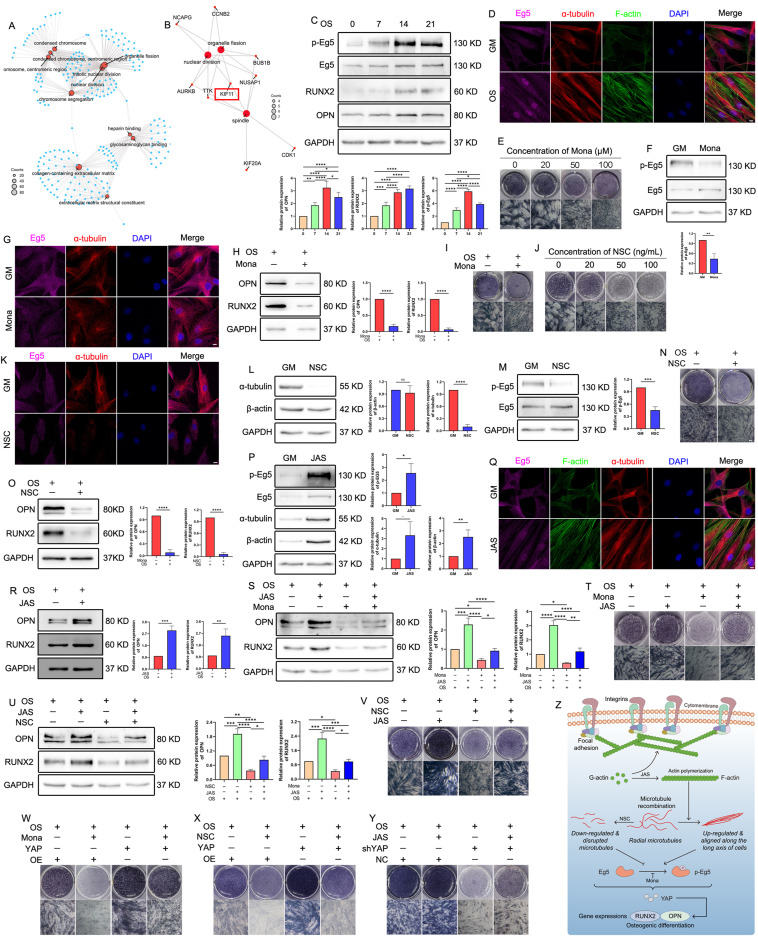
Actin polymerization regulates the osteogenesis of hASCs by influencing α-tubulin expression and Eg5 activity


Seed cells, scaffold materials, and growth factors constitute the three fundamental components of bone tissue engineering.1 In recent years, human adipose-derived stem cells (hASCs) have emerged as promising candidate cells owing to their several sources and strong differentiation ability.2 Therefore, studying the mechanisms underlying the osteogenesis of hASCs is crucial for further progress in bone tissue engineering. With the aid of bioinformatics, we conducted a comparative analysis of the differentially expressed genes between osteogenically induced and uninduced groups and identified a crucial gene, Eg5. Eg5, alternatively referred to as KIF11 or Eg5 kinesin motor protein, is a constituent of the kinesin motor protein family, exerting a substantial influence on the initial phases of cellular division through its regulation of spindle formation and functionality.3, 4, 5 Previous literature has suggested that Eg5 could generate the sliding force on the spindle pole microtubules, pushing the two extremes of the spindle away from each other and aiding in the accurate separation of chromosomes into daughter cells.5 Our team has long focused on the changes in the cytoskeletons in osteogenesis and found that microfilaments and microtubules are closely related to the osteogenesis of hASCs. Based on these findings, we hypothesized that Eg5, microtubules, and actin microfilaments might cooperate to regulate the osteogenesis of hASCs. Therefore, the present study focused on determining a potential regulatory association among Eg5, microtubules, and actin microfilaments and the direction of signal transduction across these elements. These findings will contribute novel perspectives and methodologies to inform future investigations in the field of bone tissue engineering.