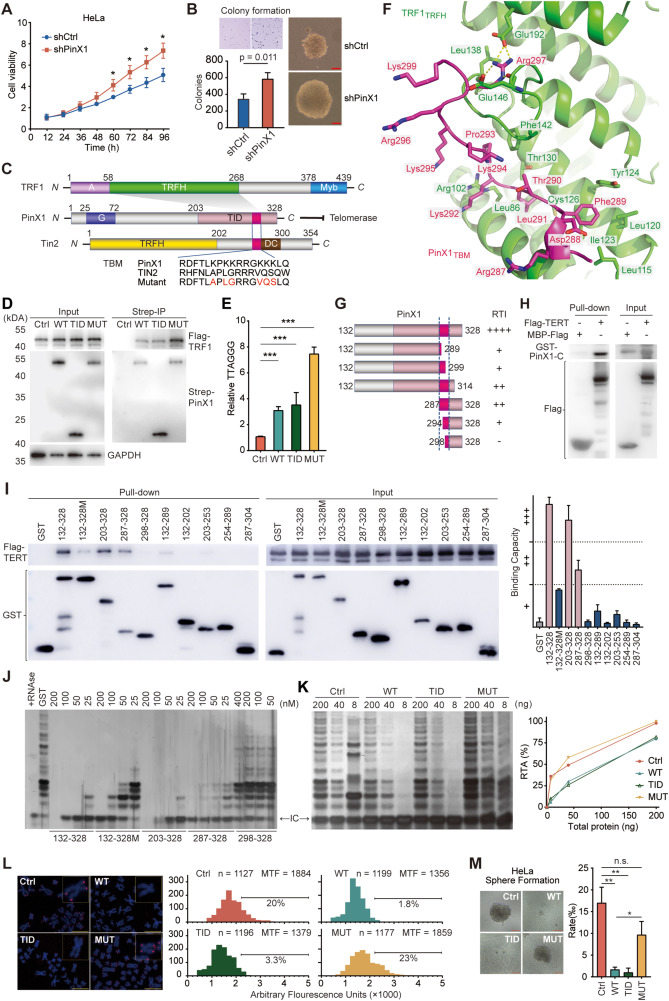
PinX1 suppresses cancer progression by inhibiting telomerase activity in cervical squamous cell carcinoma and endocervical adenocarcinoma


Telomerase plays an essential role in the immortalization and stemness of cancer cells. PIN2/TRF1-interacting telomerase inhibitor 1 (PinX1) functions as a telomerase inhibitor and tumor suppressor.1 However, the underlying mechanism is still not clear. Here, we report the molecular basis of the tumor suppression function of PinX1. We determined the crystal structure of the TRFH (TRF homology) domain of TRF1 in complex with a short TRF1-binding motif of PinX1 and revealed that PinX1 bound to TRF1 via the F-X-L-X-P motif, providing a structural basis of how PinX1 is recruited to the telomeric region by TRF1. We demonstrated that PinX1 is directly associated with and inhibits telomerase activity by TID (telomerase inhibitory domain) with crucial lysine residues clustered in PinX1292-301. In cervical squamous cell carcinoma and endocervical adenocarcinoma (CESC), which is featured by short telomeres and high telomerase activity, PinX1-TID efficiently inhibits cancer stemness traits by primarily targeting telomerase activity. These findings provide valuable insights for developing strategies to treat cancers with short telomeres and advancing telomerase inhibitor therapeutics.