Hypercalciuria switches Ca2+ signaling in proximal tubular cells, induces oxidative damage to promote calcium nephrolithiasis


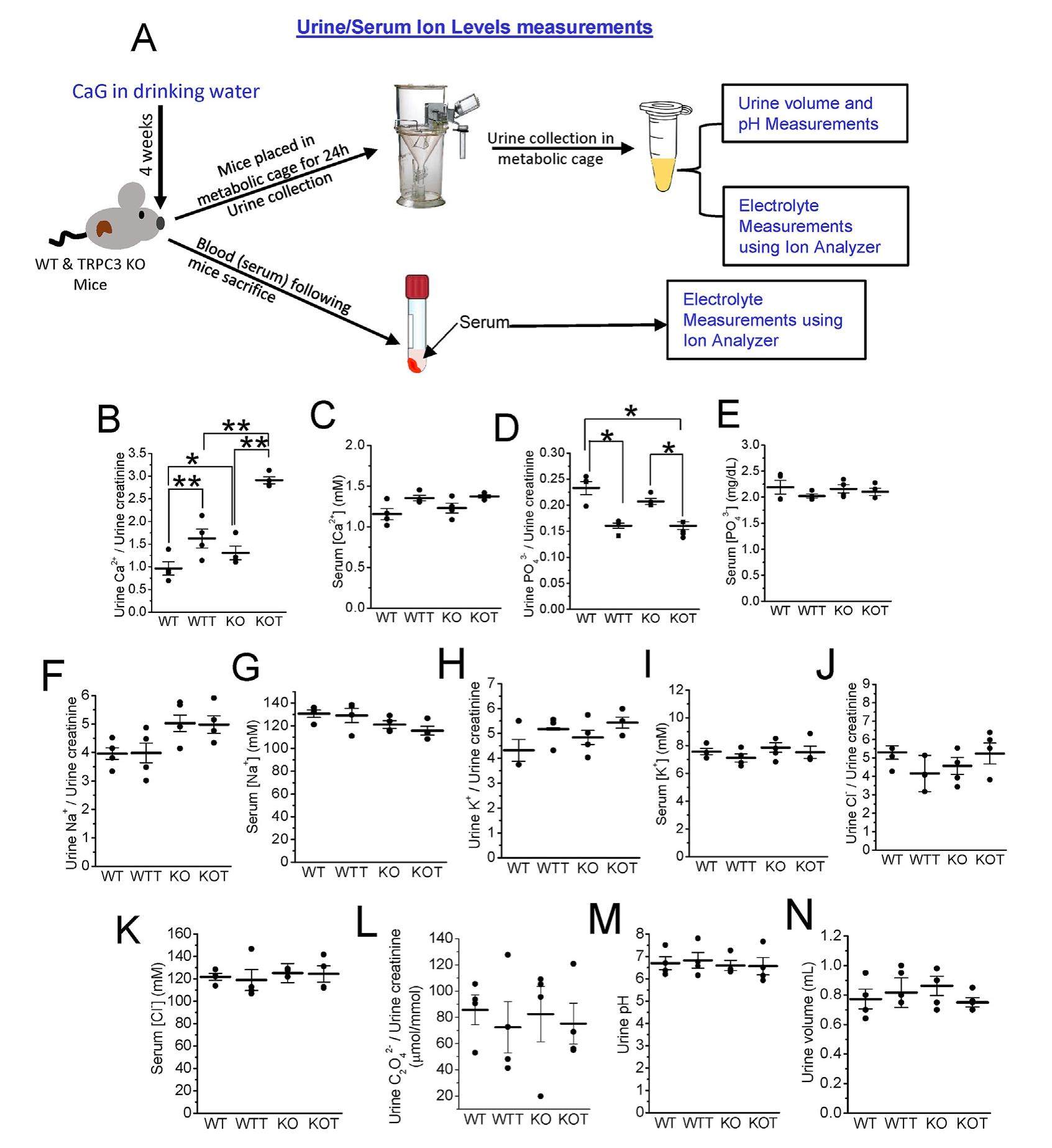
Proximal tubule (PT) transports most of the renal Ca2+, which was usually described as paracellular (passive). We found a regulated Ca2+ entry pathway in PT cells via the apical transient receptor potential canonical 3 (TRPC3) channel, which initiates transcellular Ca2+ transport. Although TRPC3 knockout (-/-) mice were mildly hypercalciuric and displayed luminal calcium phosphate (CaP) crystals at Loop of Henle (LOH), no CaP + calcium oxalate (CaOx) mixed urine crystals were spotted, which are mostly found in calcium nephrolithiasis (CaNL). Thus, we used oral calcium gluconate (CaG; 2%) to raise the PT luminal[Ca2+]o further in TRPC3-/- mice for developing such mixed stones to understand the mechanistic role of PT-Ca2+ signaling in CaNL. Expectedly, CaG-treated mice urine samples presented with numerous mixed crystals with remains of PT cells, which were pronounced in TRPC3-/- mice, indicating PT cell damage. Notably, PT cells from CaG-treated groups switched their mode of Ca2+ entry from receptor-operated to store-operated pathway with a sustained rise in intracellular[Ca2+] ([Ca2+]i), indicating the stagnation in PT Ca2+ transport. Moreover, those PT cells from CaG-treated groups demonstrated an upregulation of calcification, inflammation, fibrotic, oxidative stress, and apoptotic genes; effects of which were more robust in TRPC3 ablated condition. Furthermore, kidneys from CaG-treated groups exhibited fibrosis, tubular injury and calcifications with significant reactive oxygen species generation in the urine, thus, indicating in vivo CaNL. Taken together, excess PT luminal Ca2+ due to escalation of hypercalciuria in TRPC3 ablated mice induced surplus CaP crystal formation and caused stagnation of PT[Ca2+]i, invoking PT cell injury, hence mixed stone formation.