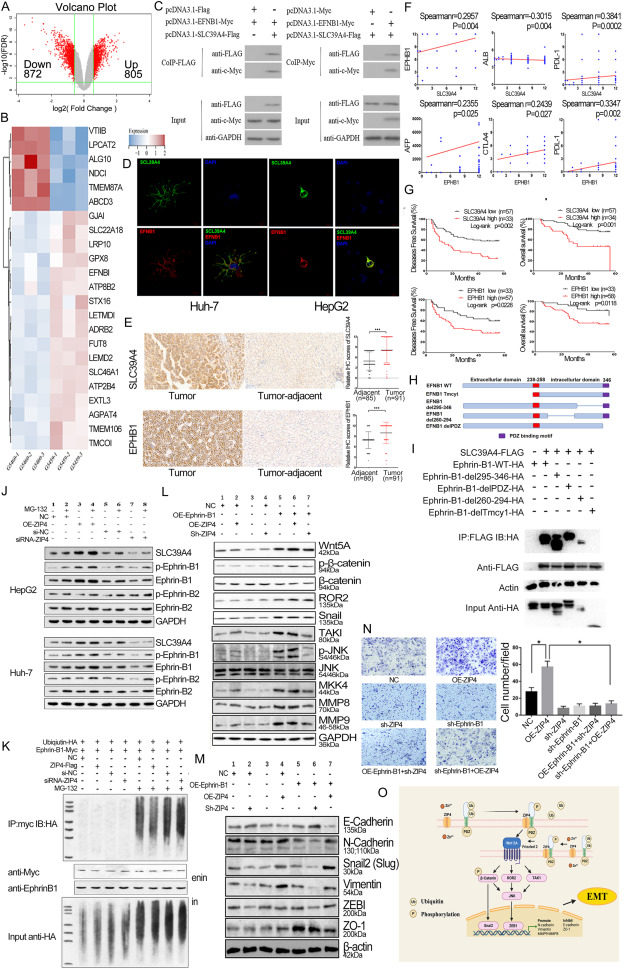
ZIP4 inhibits Ephrin-B1 ubiquitination, activating Wnt5A/JNK/ZEB1 to promote liver cancer metastasis


Hepatocellular carcinoma (HCC), a highly malignant tumor, faces a major challenge with its high post-hepatectomy recurrence impacting patient survival. Mechanisms and preventive strategies remain to be resolved. Zrt-/Irt-like protein 4 (ZIP4, also called SLC39A4/solute carrier family 39 member 4) belongs to the zinc transporter ZIP superfamily of which the members play an important role in maintaining zinc steady state, and its high expression in various tumors is associated with the prognosis of cancer patients.1 Recently, it has been reported that ZIP4 promoted the development of pancreatic cancer through the zinc finger E-box binding homeobox 1 (ZEB1)/Integrin α3β1/equilibrative nucleoside transporter (ENT1) 1,2 and zona occludens 1 (ZO-1)/claudin-1/ZEB1 pathways.3 In nasopharyngeal carcinoma, ZIP4 induces epithelial–mesenchymal transition (EMT) through the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) signaling pathway and promotes migration and invasion.4 In our previous study, ZIP4 was found to be highly expressed in HCC and promoted HCC invasion and metastasis.5 But so far, the direct regulation mechanism for ZIP4 promotes liver cancer is still unclear. In this study, we found that ZIP4 binds to Ephrin-B1 to inhibit the ubiquitination of Ephrin-B1, regulating the Wnt family member 5A (Wnt5A)/Jun N-terminal kinase (JNK)/ZEB1 signaling pathway and promoting EMT, thereby inducing invasion and metastasis of liver cancer cells.