Transcriptional programs associated with luminal play a vital role in invasive mucinous lung adenocarcinoma


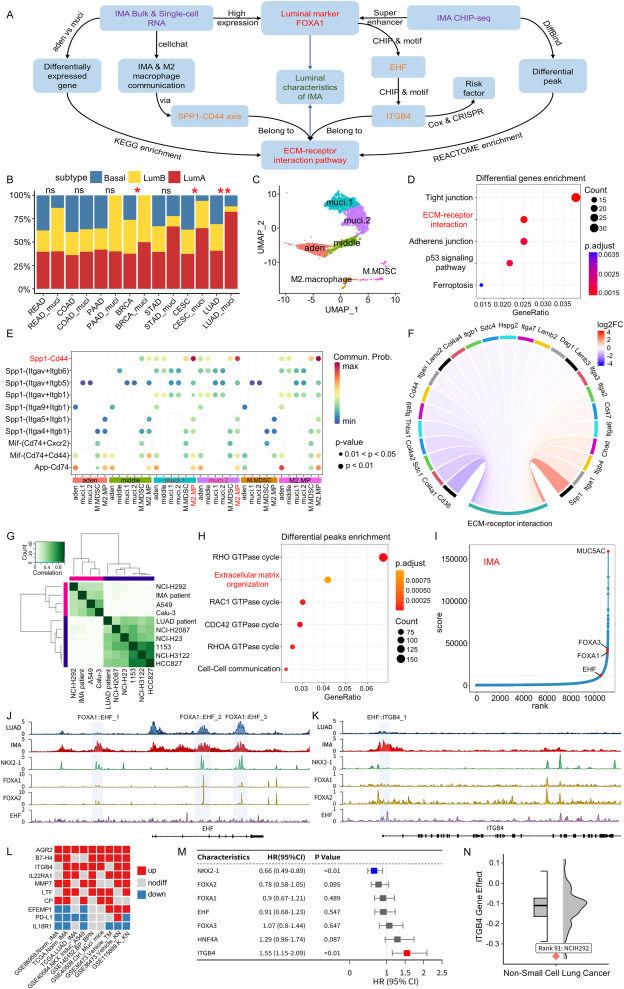
Invasive mucinous lung adenocarcinoma (IMA) is an aggressive subtype characterized by the presence of tumor cells with goblet cell morphology and abundant intracytoplasmic mucin. A previous study had shown that all epithelial tumors share similar gene expression-based luminal/basal subtypes and can impact treatment response.1 We identified the subtype of mucinous adenocarcinoma and found that many of them exhibit a luminal phenotype, particularly IMA. We established a single-cell atlas of the transition from lung adenocarcinoma (LUAD) to IMA and found that the luminal phenotype of IMA is characterized by high expression of FOXA1 and specific enrichment of the extracellular matrix (ECM)-receptor interaction pathway. CellChat analysis revealed that the SPP1-CD44 axis mediated communication between IMA and M2 macrophages. By chromatin immunoprecipitation sequencing analysis, we observed consistent enrichment of differential histone modifications at the ECM pathway. The luminal subtype marker FOXA1 is central to the luminal-associated transcriptional programs and may bind to super-enhancer regions near EHF and promote its expression. Furthermore, EHF can bind to the transcription start site region of the prognostic risk factor ITGB4 and promote its expression. Overall, the luminal-associated transcriptional programs (FOXA1-EHF-ITGB4) and its downstream ECM-receptor interaction pathway (SPP1, CD44, ITGB4) play a crucial role in IMA, influencing its immunity and tumor risk (Fig. 1A).