In-depth profiling of tumor tissue derived from malignant pleural mesothelioma patients identifies potential biomarkers predicting response to immune-checkpoint inhibitor therapy


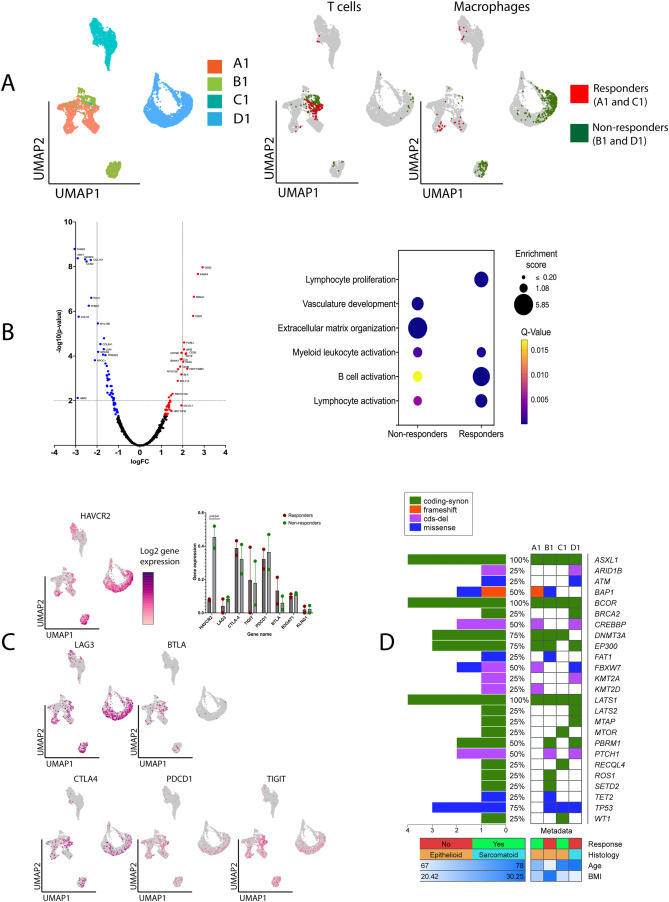
Malignant pleural mesothelioma (MPM) is a rare and aggressive cancer with low survival probability as it is generally diagnosed at later stages.1 Using a combination of immune-checkpoint inhibitors (ICIs) ipilimumab (IPI) and nivolumab (NIVO) as a first-line treatment for unresectable MPM, the CheckMate 743 trial reported higher overall survival and prolonged duration of response compared with traditional chemotherapy.1 This combination has recently been approved as a new first-line standard of care for patients with advanced MPM, although the incidence of immune-related adverse events reached 80 %, with 31% of patients experiencing grade 3-4 immune-related adverse events (irAEs).1 The unpredictable nature and severity of immune-related adverse events emphasize a critical need to conduct in-depth translational studies to characterize the tumor microenvironment and systemic immune response that contribute to the efficacy and safety outcomes of ICI therapy.