A homozygous nonsense mutation in DNAJC30 causes Leber's hereditary optic neuropathy with Leigh-like phenotypes


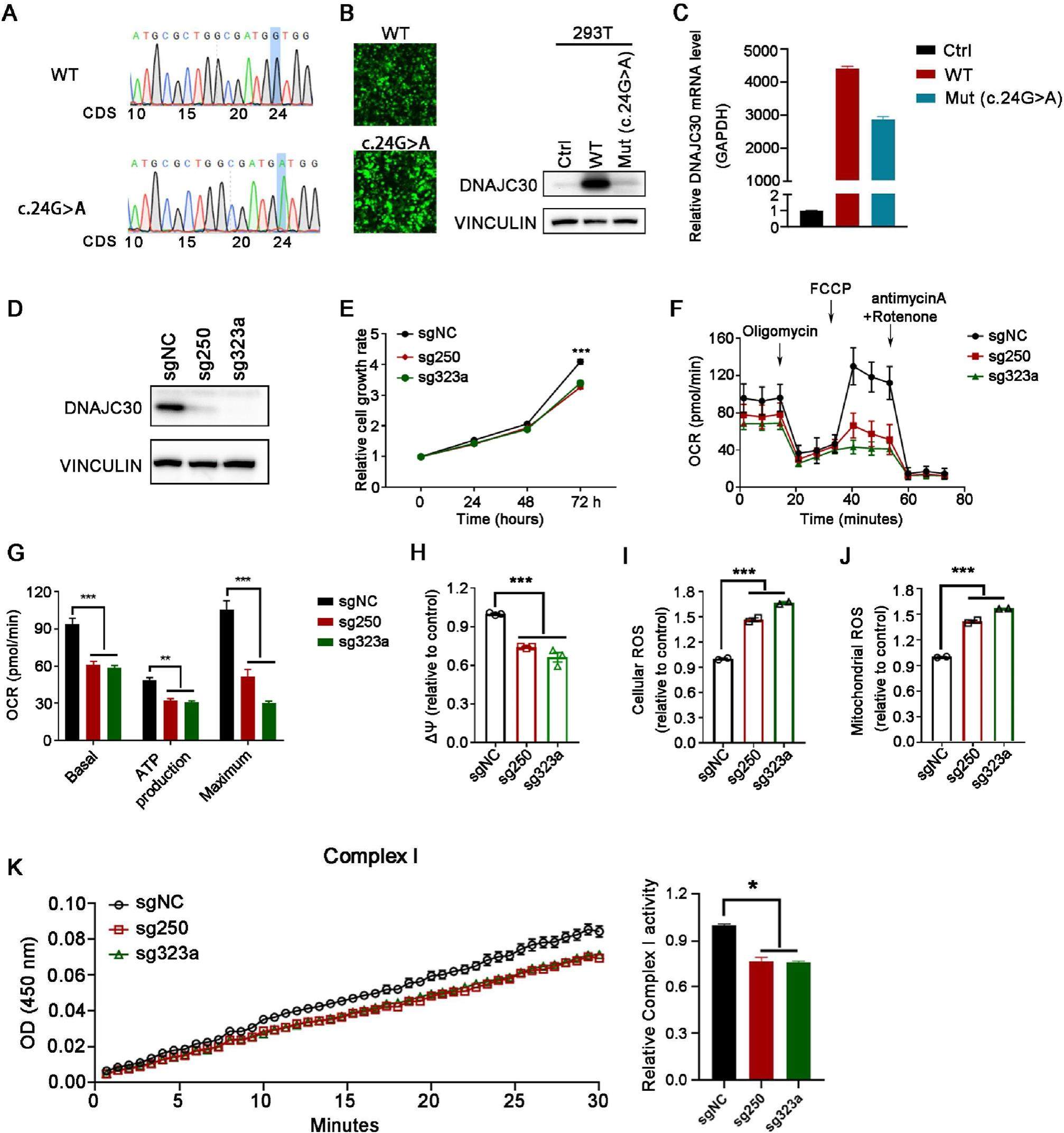
Nuclear encoded genes can cause early-onset mitochondria-related disorders such as Leigh or Leigh-like syndrome. Defects in DNAJC30 have been implicated in mitochondriarelated diseases such as Leber's hereditary optic neuropathy (LHON) and Williams syndrome (WS). However, the role of DNAJC30 in disease progression concerning mitochondrial dysfunction has yet to be fully understood. Here we report a 12-year-old boy with acute dystonia onset at age 10.Brain magnetic resonance imaging (MRI) showed bilateral basal ganglion and thalamic hyperintensities concerning putaminal necrosis. He then developed bilateral optic atrophy and rapid progressive bilateral visual loss. Exome sequencing analysis of his peripheral blood sample revealed a homozygous nonsense germline variant, c.24G> A (p. W8X) in the DNAJC30 gene, proven to result in a complete loss of DNAJC30 protein expression in cells. Remarkably, the same germline variant was then identified by whole genome sequencing in another unrelated patient, a 17-year-old male. He also showed acute bilateral optic atrophy, subacute central vision loss, and abnormal brain MRI.In vitro functional analysis further confirmed that DNAJC30 deletion inhibited cell growth and induced mitochondrial disorders, evidenced by decreased oxygen consumption rate (OCR), reduced mitochondrial membrane potential (ΔΨm), and increased cellular and mitochondrial reactive oxygen species (ROS), presumably due to decreased complex I enzyme activity. Collectively, a homozygous nonsense germline variant in DNAJC30 (c.24G> A) was identified to cause mitochondria-related disorders. This rare DNAJC30 pathogenic variant will be useful in their diagnosis/prognosis and highlights the significance of the roles of DNAJC30 in the maintenance of normal mitochondrial function and brain development.