Otof gene transfer in DFNB9 mice carrying human founder non-truncating alleles


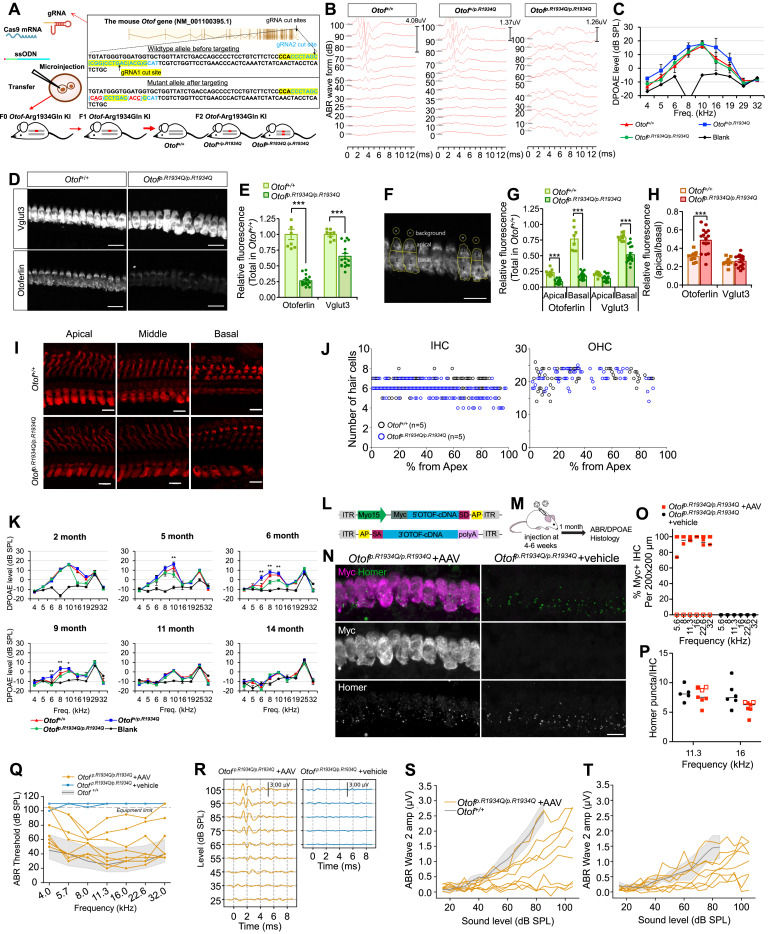
Gene therapy has shown promise for treating sensorineural hearing loss, supported by numerous successful preclinical studies. From the perspective of translation to humans, researchers have focused more on the genetic causes of profound sensorineural hearing loss, where the sensory epithelium remains viable and intact for a considerable time after birth in humans. A key human deafness gene that best fits such a context is OTOF (GenBank AF183185.1), of which protein products, otoferlin, is essential for synaptic exocytosis and vesicle replenishment at the inner hair cell level in the cochlea.1 Several preclinical studies where Otof cDNA was transferred into the cochlea of adult Otof null allele mice, including both OtofΔ/Δ and Otofp.Q939∗/p.Q939∗, successfully achieved near-normal thresholds,2 and furthermore, relatively successful human clinical trials of gene therapy have been conducted for DFNB9 (OMIM 60381) patients showing auditory neuropathy.3,4 One important consideration is that, to date, there has not been a single case in any preclinical study or human clinical trial where both alleles of OTOF/Otof were non-truncating. However, many human patients with DFNB9 carry non-truncating missense variants in OTOF, indicating that their inner hair cells express some mutant RNA/proteins. This raises questions about how these mutant RNA/proteins might interact with exogenous transgene products, affect the function of newly expressed otoferlin, and influence the outcome of gene therapy. To address this, research on wild-type Otof cDNA transfer in mouse models with two copies of common founder missense variants, like p.Arg1939Glu (p.R1939Q), prevalent in Korean and Japanese populations,5 is essential.