PGC-1α promotes mitochondrial respiration and biogenesis during the differentiation of hiPSCs into cardiomyocytes


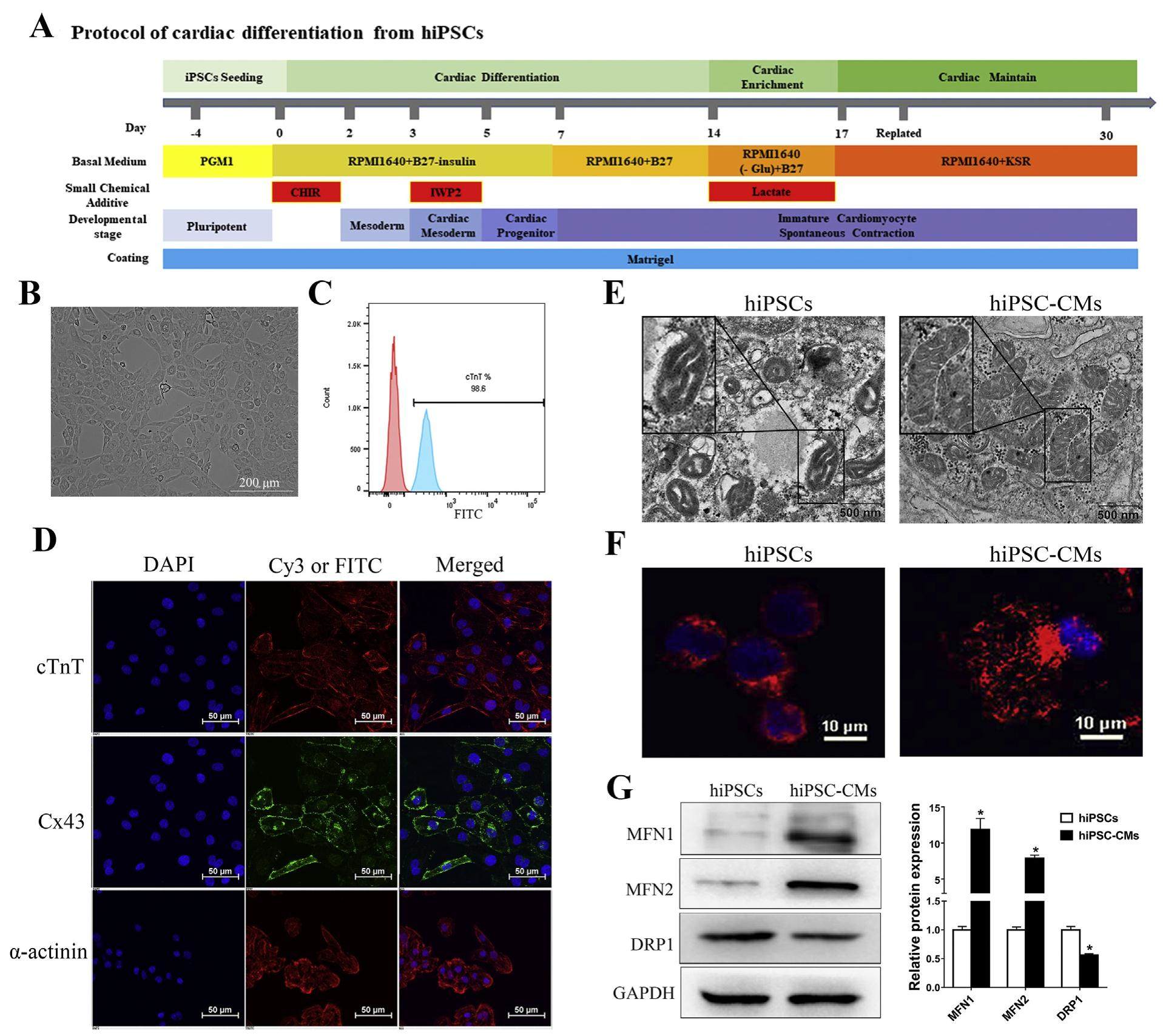
Although it is widely accepted that human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) are readily available, robustly reproducible, and physiologically appropriate human cells for clinical applications and research in the cardiovascular field, hiPSC-CMs cultured in vitro retain an immature metabolic phenotype that limits their application, and little is known about the underlying molecular mechanism controlling mitochondrial metabolic maturation during human induced pluripotent stem cells (hiPSCs) differentiation into cardiomyocytes. In this study, we found that peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) played an important role in inducing mitochondrial biogenesis and establishing oxidative phosphorylation (OXPHOS) during the cardiac differentiation of hiPSCs. Knocking down PGC-1a by siRNA impaired mitochondrial respiration, while upregulating PGC-1α by ZLN005 promoted mitochondrial biosynthesis and function by regulating the expression of downstream genes involved in mitochondrial dynamics and oxidative metabolism in hiPSCCMs. Furthermore, we found that estrogen-related receptor α (ERRα) was required for the induction of PGC-1a stimulatory effects in hiPSC-CMs. These findings provide key insights into the molecular control of mitochondrial metabolism during cardiac differentiation and may be used to generate more metabolically mature cardiomyocytes for application.