THBS1 regulates trophoblast fusion through a CD36-dependent inhibition of cAMP, and its upregulation participates in preeclampsia


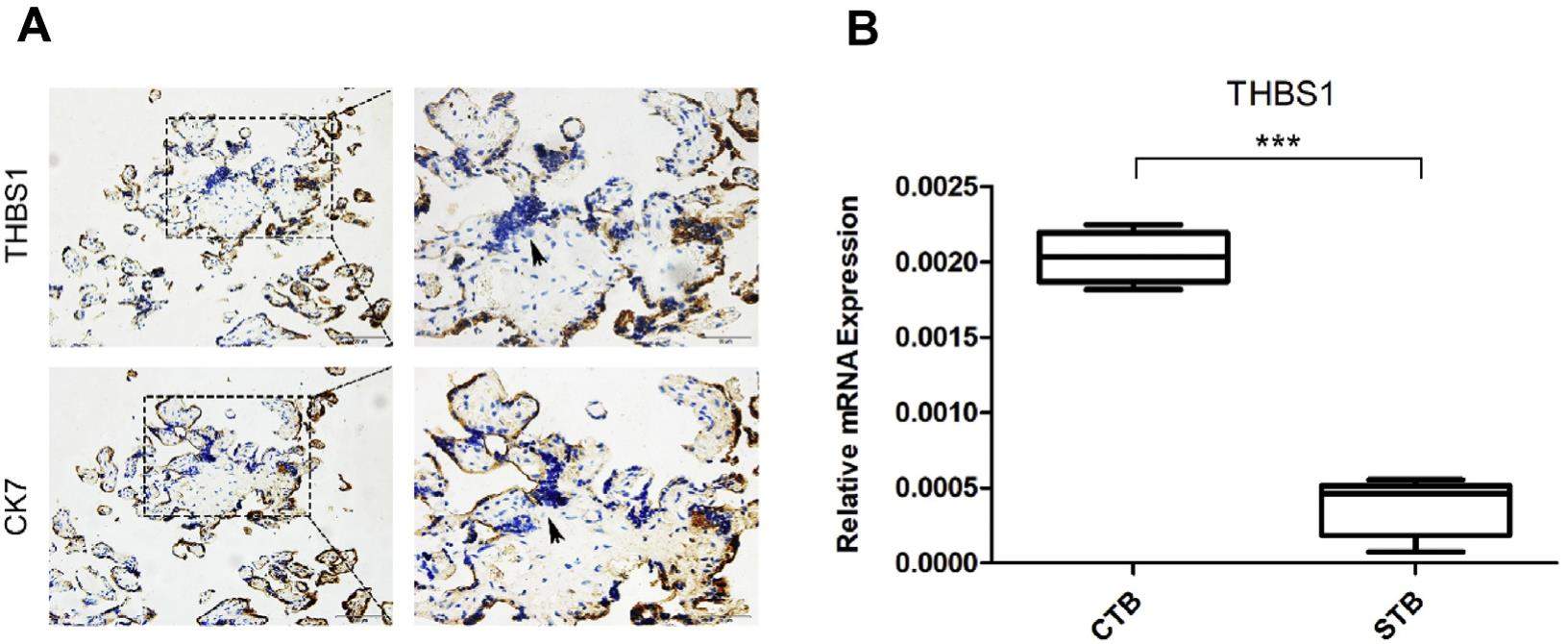
Preeclampsia is a pregnancy complication which threatens the survival of mothers and fetuses. It originates from abnormal placentation, especially insufficient fusion of the cytotrophoblast cells to form the syncytiotrophoblast. In this study, we found that THBS1, a matricellular protein that mediates cell-to-cell and cell-to-matrix interactions, is downregulated during the fusion of primary cytotrophoblast and BeWo cells, but upregulated in the placenta of pregnancies complicated by preeclampsia. Also, THBS1 was observed to interact with CD36, a membrane signal receptor and activator of the cAMP signaling pathway, to regulate the fusion of cytotrophoblast cells. Overexpression of THBS1 inhibited the cAMP signaling pathway and reduced the BeWo cells fusion ratio, while the effects of THBS1 were abolished by a CD36-blocking antibody. Our results suggest that THBS1 signals through a CD36-mediated cAMP pathway to regulate syncytialization of the cytotrophoblast cells, and that its upregulation impairs placental formation to cause preeclampsia. Thus, THBS1 can serve as a therapeutic target regarding the mitigation of abnormal syncytialization and preeclampsia.