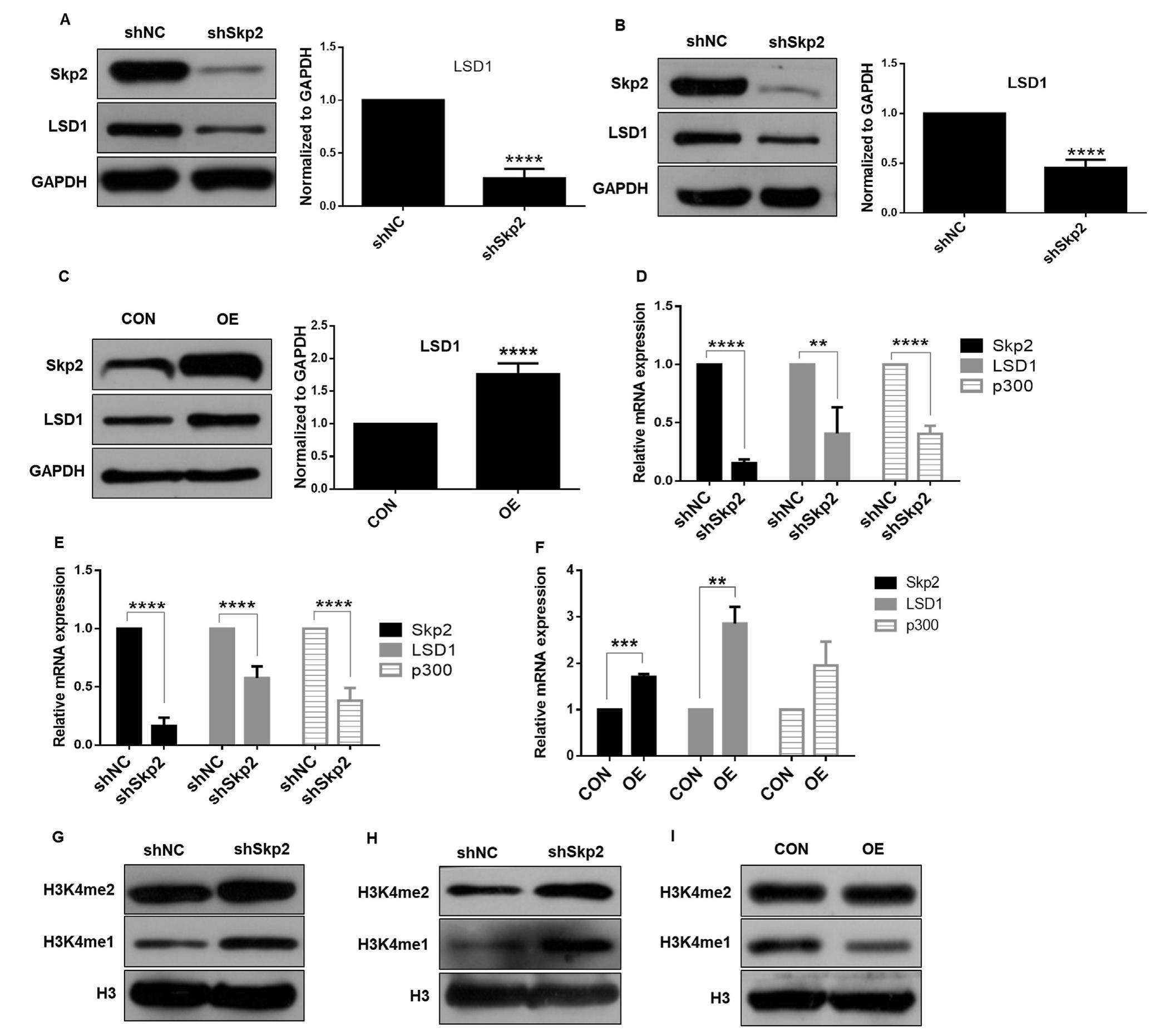
Skp2 is a novel regulator of LSD1 expression and function in gastric cancer


S-phase kinase-associated protein 2 (Skp2) is a well-characterized oncoprotein localized mainly in the nucleus and cytoplasm. It is an integral component of SCFSkp2 E3 ubiquitin ligase complex which confers substrate selectivity to the ligase by specifically targeting a distinct set of proteins destined for proteasomal degradation such as p21, p27, cyclin E, and c-Myc. Skp2 is crucial in a multitude of cellular processes including cell cycle, cell proliferation, apoptosis, differentiation, and survival. However, despite its immense and well-established role in ubiquitin-proteasome system-mediated protein turnover, much is unknown about the function of Skp2 independent of the ubiquitination pathway. Previously, Skp2 has been reported to regulate RhoA gene transcription and the p300 signaling pathway in an E3 ligase-independent manner. Moreover, Skp2 also acts as a cofactor for c-Myc-regulated gene expression.