Targeted down-regulation of Hipp1 ameliorates tau-induced deficits in Drosophila melanogaster


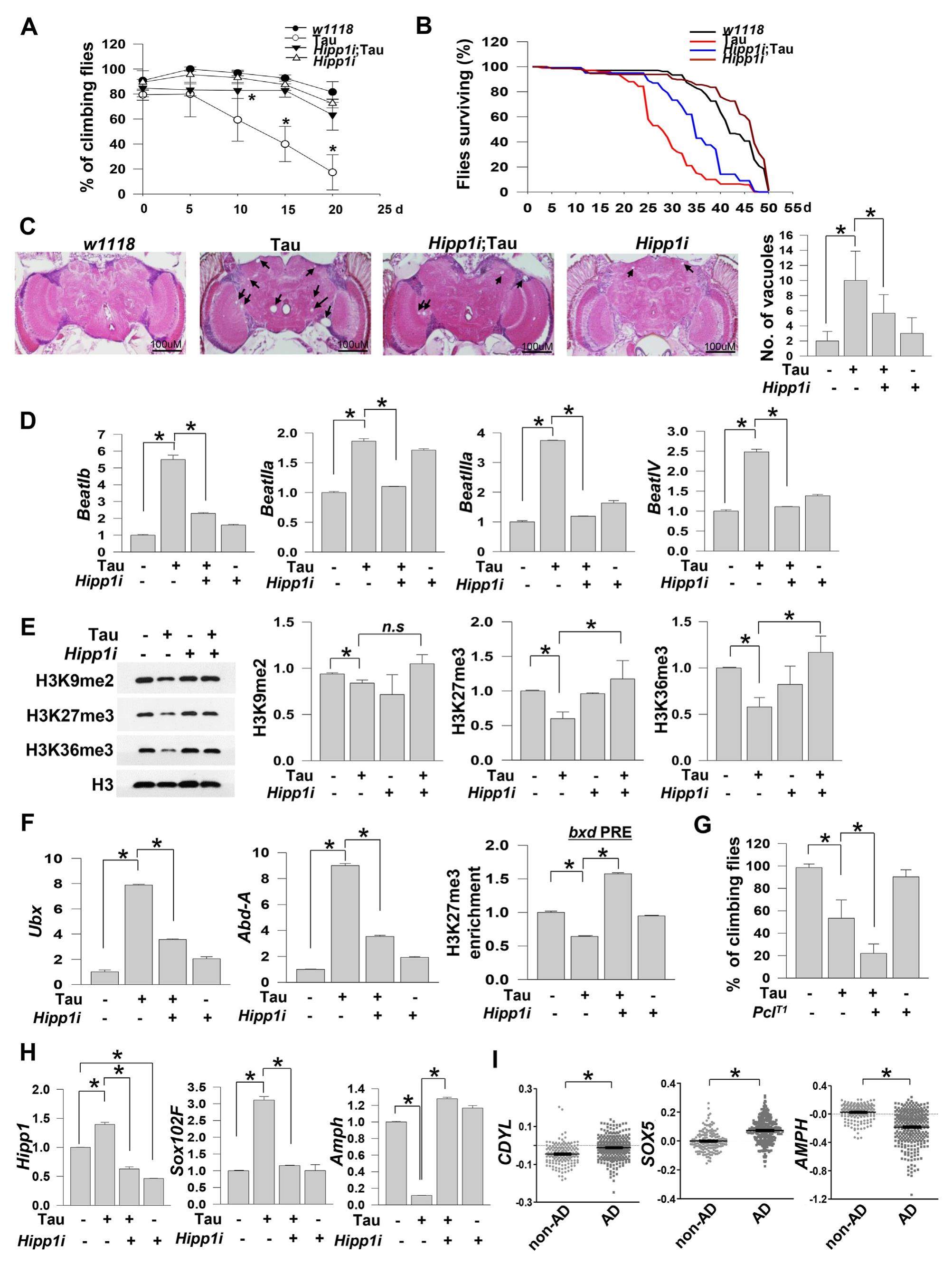
Tauopathies, such as Alzheimer's disease (AD), are neurodegenerative diseases characterized by the deposition of neurofibrillary tangles comprising hyperphosphorylated tau protein in the human brain. Given that abnormal epigenetic alterations in heterochromatin configuration have been documented in AD patients and transgenic animal models of AD, we investigated the roles of novel heterochromatin-associated interactors in tauopathies. Using transgenic flies via UAS-Gal4 binary system, we found that knockdown of Hipp1 (HP1a and insulator partner protein-1) ameliorates tauR406W (referred to as "tau" hereafter for simplicity) -induced locomotion defects, reduced lifespan, and degeneration of brain tissues. Intriguingly, Hipp1 knockdown restored tau-driven aberrant expression of putative insulator targets and aberrant insulator-mediated epigenetic alterations. HIPP1 may have a role as an insulator-binding partner regarding being implicated in tauinduced neurodegeneration. Moreover, Hipp1 knockdown in flies overexpressing tau restored the aberrant expression of AD susceptibility genes. These observations provide new insights into the roles of insulator proteins in tauopathies.