NRF2 participates in the suppressive tumor immune microenvironment of KRAS/KEAP1 co-mutant non-small cell lung cancer by inhibiting the STING pathway


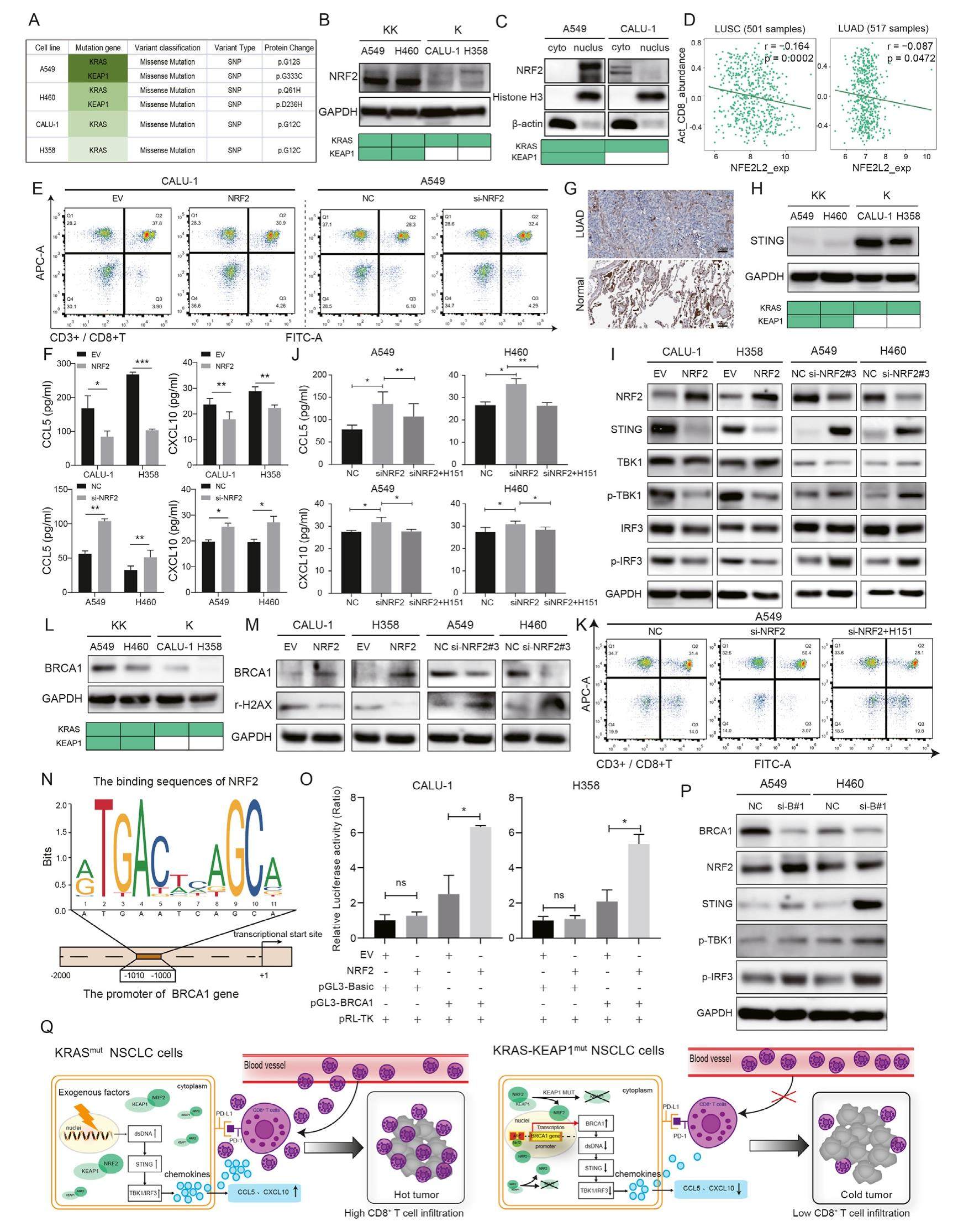
Kirsten rat sarcoma (KRAS) mutant non-small cell lung cancer (NSCLC) hasdistinctco-mutationalprofiles. Approximately 20% of KRAS mutant NSCLC cases underwent loss-of-function mutation of Kelch-like ECH-associated protein 1 (KEAP1) gene, namely KRAS-KEAP1 comutation (KK) type, which exhibited a poorer immune checkpoint inhibitor (ICI) response compared with individual KRAS mutation (K) type tumors. Recent studies suggest that K-type tumors are referred to as "hot tumors" while KK-type tumors are as "cold tumors". Therefore, clarifying the molecular mechanism of the poor ICI-responsive phenotype of KK-type tumors is the key to improving the clinical efficacy and breaking through the therapy bottleneck. Co-mutations could regulate the immune contexture to reshape the tumor immune microenvironment (TIME), which has been identified as a major influence factor of ICI response. KK-type tumors were confirmed to contain few tumor-infiltrating lymphocytes, especially CD8+ T cells, leading to a suppressive TIME, which is also defined as a "cold tumor". Here, we demonstrated that NRF2, the target of KEAP1, exerted its role as a negative regulator of CD8+ T cells recruitment by decreasing CCL5 and CXCL10 chemokines in KK-type NSCLC. Mechanistically, NRF2 promoted the transcription and expression of BRCA1 to repair DNA damage, resulting in STING pathway inactivation. We propose the combination of NRF2 inhibitor or STING agonist with ICI may be a promising therapeutic approach for patients with KK-type NSCLC.