Bone Morphogenic Protein 9 (BMP9) /Growth Differentiation Factor 2 (GDF2) modulates mouse adult hippocampal neurogenesis by regulating the survival of early neural progenitors


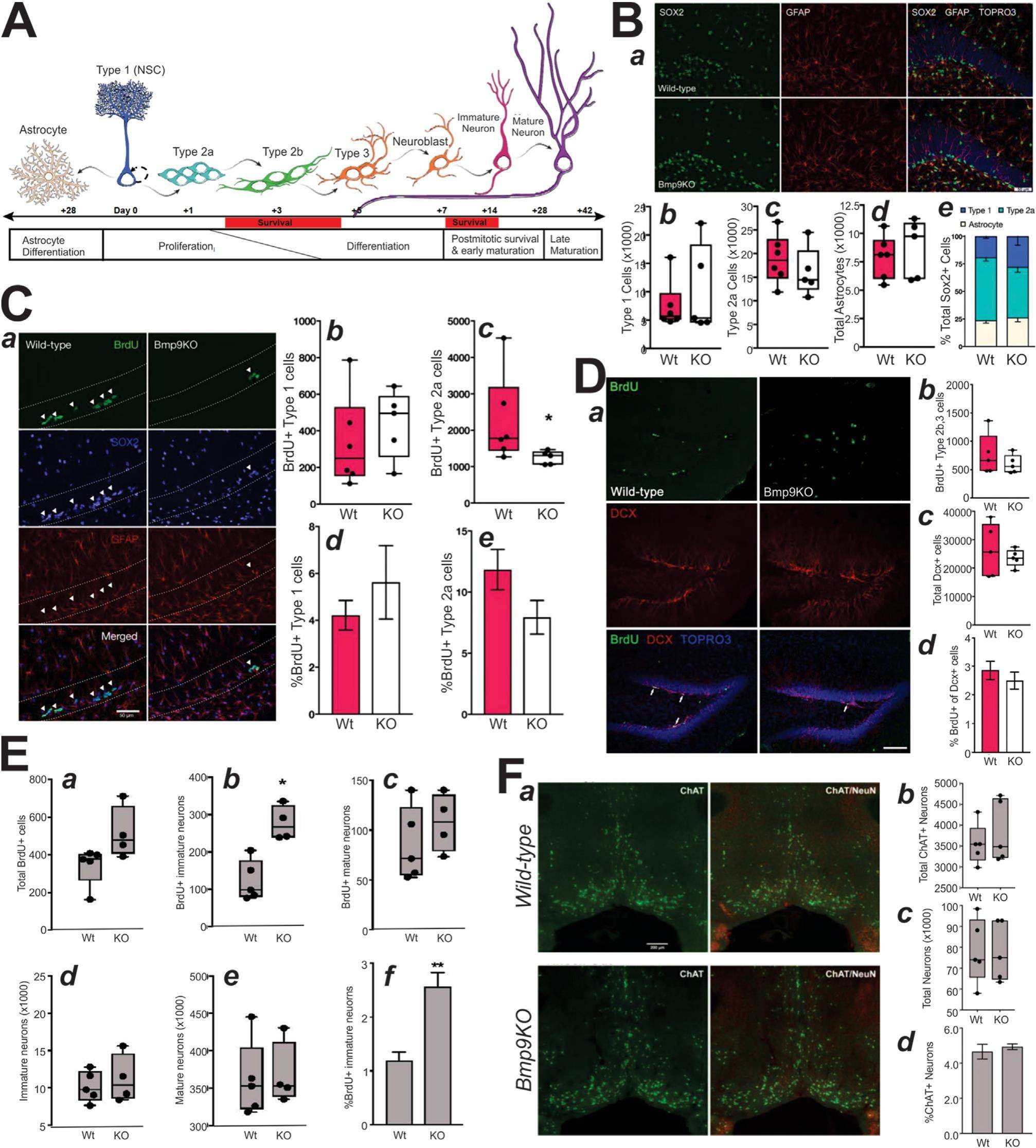
Adult neurogenesis occurs in two specialized regions of the mammalian brain, the subventricular zone (SVZ) and the subgranular zone (SGZ) of the dentate gyrus (DG). Adult hippocampal neural stem cells (NSCs), referred to as Type 1 cells represented by radial glia-like cells (RGLs), generate Type 2 cells that are divided into Type 2a and Type 2 b subpopulations, the latter of which give rise to Type 3 cells (neuroblasts). Type 3 cells exit the cell cycle and give rise to immature neurons that subsequently mature into functional granule cells. The neural regenerative capacity depends on maintaining a reservoir of NSCs and the integrity of the neurogenic niche, which are regulated by neurotransmitters and numerous signals including Notch, Wnt, and BMPs. Here we showed that BMP9 (a.k.a., GDF2) deficiency altered the dynamics of adult hippocampal neurogenesis as proliferative activity was decreased in Type 2a cells in Bmp 9 knockout (Bmp9KO) mice. Surprisingly, the number of cells committed to the neuronal lineage was comparable to wildtype while the label retention was increased in immature neurons, suggesting mitigation of reduced proliferation in Bmp9KO mice by increased cell survival. We also found Bmp9 was not expressed in the adult brain, precluding cell-autonomous regulation of neurogenic populations like other BMPs. Our findings thus demonstrate BMP9 regulates adult homeostatic neurogenesis.