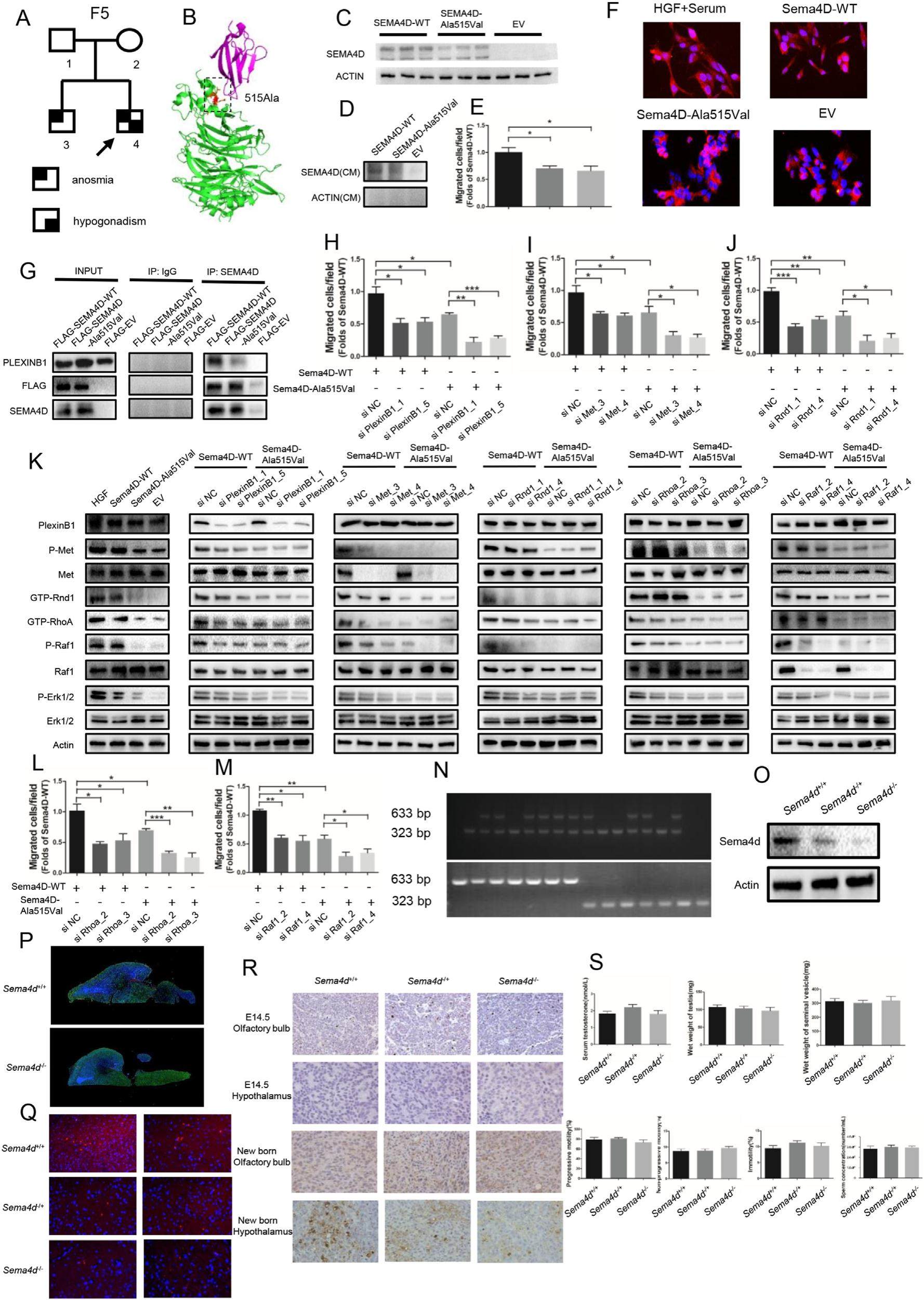
SEMA4D acts as a novel oligogenic pathogenic gene of idiopathic hypogonadotropic hypogonadism through the PlexinB1/MET/RND1/RHOA/RAF1/MAPK signaling axis


Idiopathic hypogonadotropic hypogonadism (IHH) is a rare genetic disease with clinical and genetic heterogeneity. This study aimed to investigate a novel causal gene of IHH and a homozygous mutation (p.Ala515Val) in SEMA4D, and sought to determine the mechanism of SEMA4D promoting GnRH neurons migration. The detailed materials and methods were shown in Supplementary Methods. Combination of bioinformatics, in silico analysis and in vitro analysis indicated the homozygous mutation as a loss-of-function mutation. Functional experiments were conducted to explore SEMA4D modulating GN11 cells migration through SEMA4D/PlexinB1/Met/Rnd1/RhoA/Raf1/MAPK signaling pathway. The results of in vivo experiment demonstrated the reduced population of GnRH neurons at the hypothalamus in Sema4D-/-mice models with normal serum testosterone level, reproductive system, and quality of sperm, consistent with the oligogenic pathogenicity of IHH. In this study, we expanded the genetic spectrum of IHH, and provided theoretical basis for genetic diagnosis and personalized treatment of IHH patients.