Downregulated nuclear lncRNA NRON inhibits SHP2/Wnt/β-catenin signaling and cardiomyocyte differentiation during the development of Tetralogy of Fallot


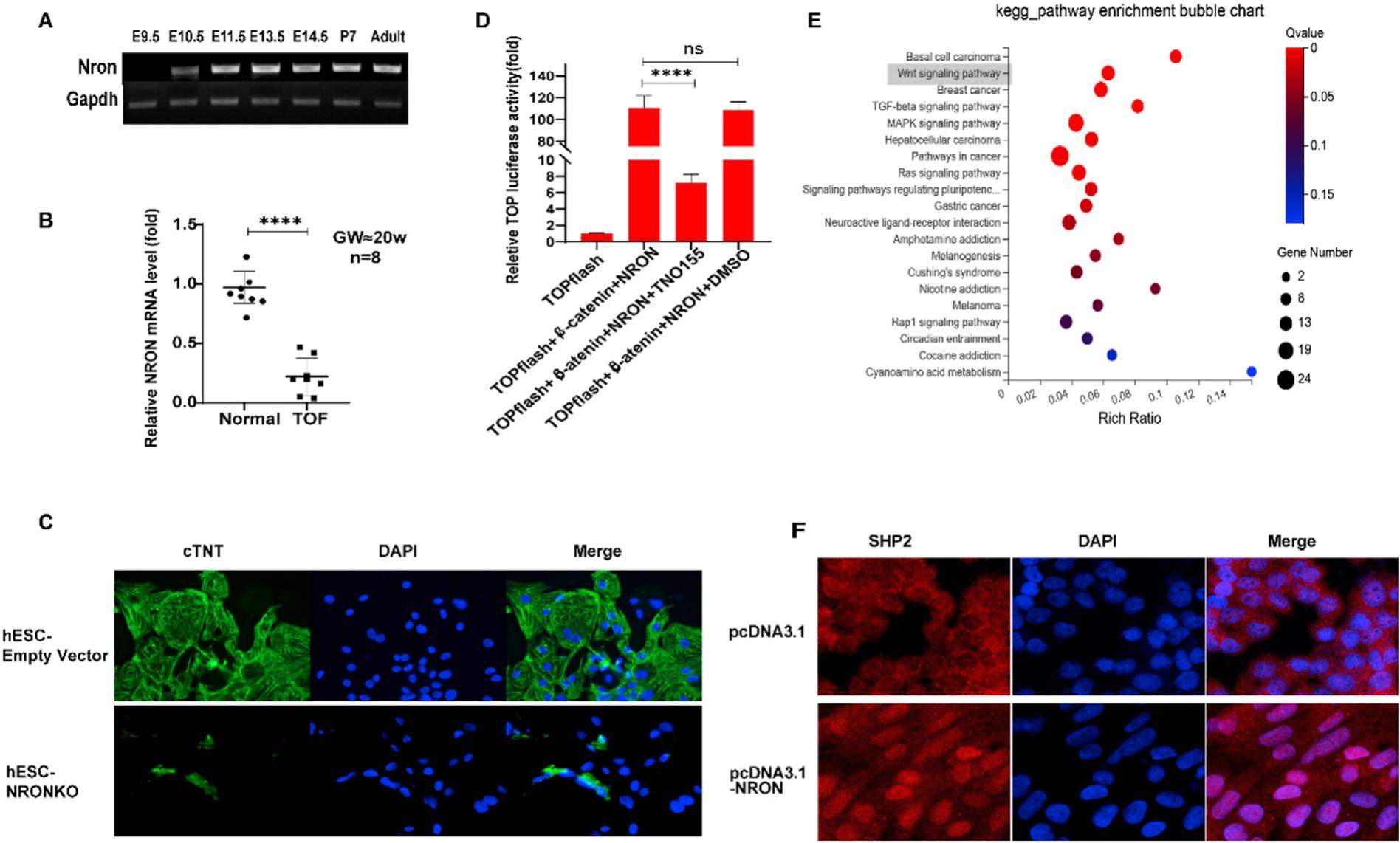
Tetralogy of Fallot (TOF) is the most common cyanotic congenital heart disease and the incidence of late cardiac death in long-term survivors continues to increase. So, there is an urgent need to explore the etiology and pathogenesis of TOF. The precise cause of TOF is currently unclear, and exploration of the pathogenesis has focused increasingly in recent years on the roles of noncoding gene products, especially long noncoding RNAs (lncRNAs). The lncRNA NRON is localized at the 9q33.3 revered strand. The related literature has revealed that its transcript is enriched in human cardiac muscle and NRON over-expression has been reported to reverse epithelial-to-mesenchymal transition (EMT) which is known to contribute to valve development in the heart. Based on these findings, we hypothesized that abnormal expression of NRON was probably relevant to congenital heart defects. This study investigated the role of lncRNA NRON in the nucleus and its correlation with cardiac development and TOF.