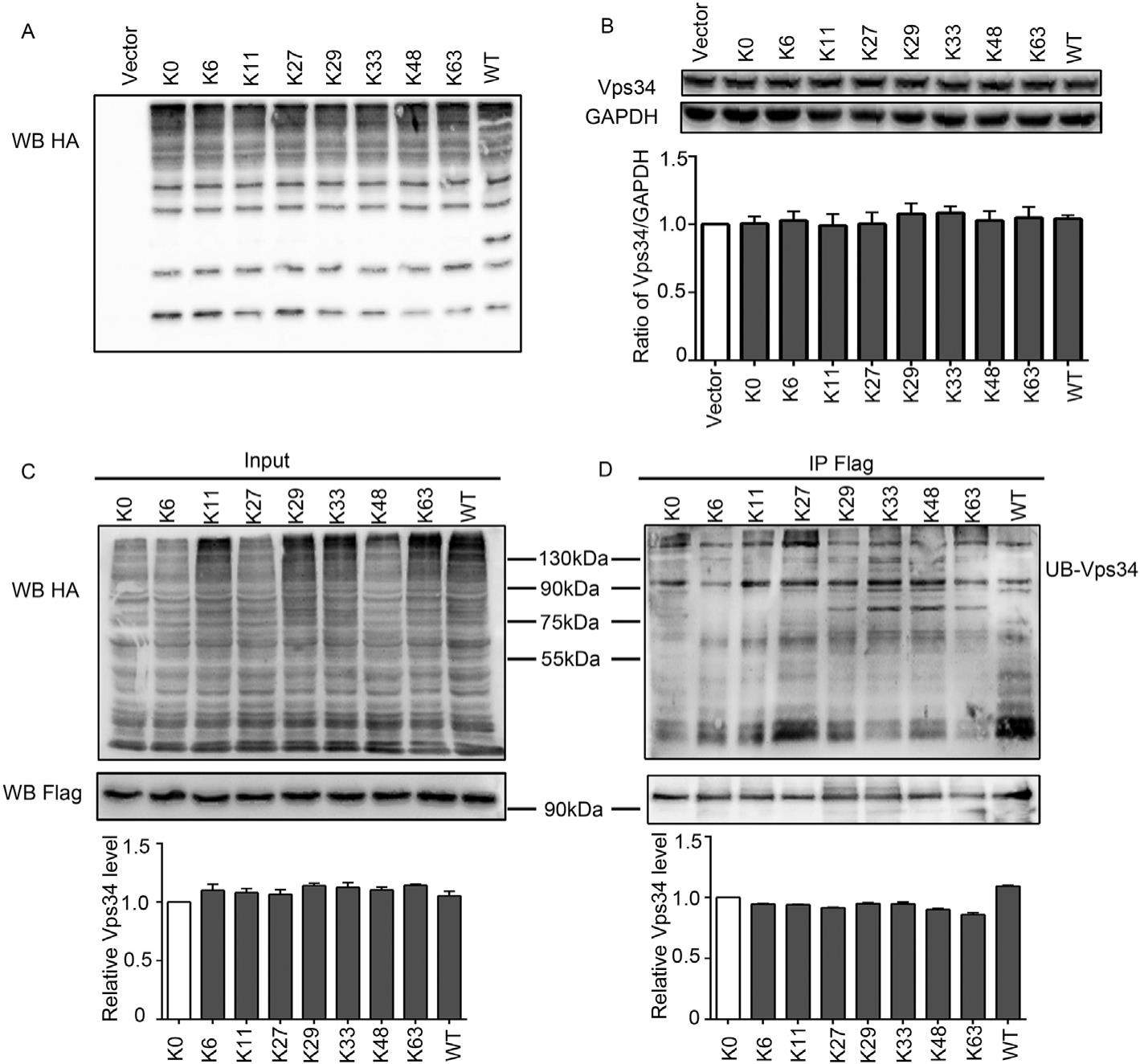
Ubiquitination status does not affect Vps34 degradation


Vps34 (vacuolar protein-sorting 34) plays important role in autophagy and endosomal trafficking. These processes are closely associated protein ubiquitination and degradation. We have hypothesized that Vps34 ubiquitination status would also control its degradation. Here, we report that our results did not support this assumption. In cells transiently transfected with ubiquitin (UB) constructs contained different lysine residues (Ks), Vps34 ubiquitination could occur regardless of the presence of any Ks in UB. However, Vps34 protein levels were not significantly altered in cells transiently transfected with these UB mutants. We further found that Vps34 protein was altered by pharmacological manipulation of E2/E3 activity; yet this effect was not significantly affected by UB overexpression. In vivo experiments revealed that in APP/PS1 mice, an animal model of Alzheimer's disease (AD), although ubiquitination of Vps34 was significantly reduced, Vps34 protein levels remained unchanged. Vps34 indeed was subjected to proteasomal or lysosomal degradation, as prolonged treatment of proteasomal inhibitor MG132 or lysosomal inhibitor chloroquine elevated Vps34 protein levels. We conclude that unlike most of other proteins, Vps34 ubiquitination is not closely associated with its degradation.