Elevated miR-34a induced by lipotoxicity and inflammation mediates pathophysiological communication between hepatocytes and hepatic stellate cells in liver fibrosis


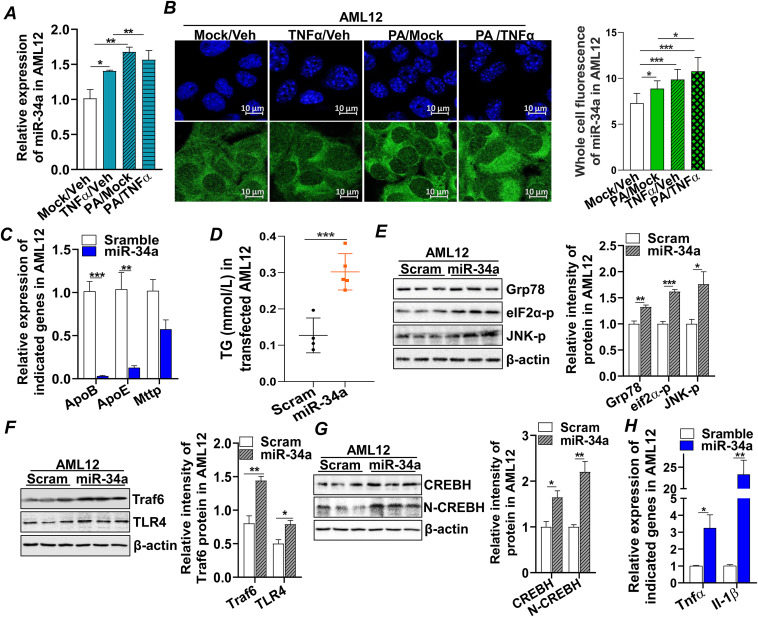
Increased mortality in patients with metabolic dysfunction-associated steatotic liver disease (MASLD) imposes an urgent need to elucidate the pathogenesis of MASLD so that novel therapeutic strategies may be identified. Here, we delineate the mechanism of microRNA-34a-5p (miR-34a) in the progressive liver injury of MASLD and liver fibrosis. Specifically, liver tissue from patients with obesity-associated hepatic steatosis, metabolic dysfunction-associated steatohepatitis (MASH), and fibrosis, as well as liver tissues from a human MASLD-like mouse model, were utilized for this study. We found that lipotoxicity resulting from obesity or saturated free fatty acid treatment induced miR-34a expression in human liver tissue or mouse hepatocytes, which was accompanied by dysregulation of lipoprotein metabolism, activation of inflammation, and ballooning degeneration of hepatocytes. Moreover, increased cellular miR-34a induced by treatment with saturated fat, palmitic acid, or transfection of miR-34a mimic was released from injured hepatocytes into the conditional cell culture media, which mediated pathological communications between hepatocytes and hepatic stellate cells, activated pro-fibrogenic signaling in hepatic stellate cells, and induced extracellular matrix remodeling. These phenotypes were recapitulated in a human MASLD-like mouse model in which MASLD and liver fibrosis were induced via streptozotocin treatment and high-fat feeding. Elevated expression of miR-34a was found in mouse liver tissues, which conveyed the progressive liver injury from steatosis to MASH and liver fibrosis. Our findings demonstrate that elevated miR-34a induced by lipotoxicity and metabolic inflammation are key driving factors in the progressive liver injury from simple steatosis to MASH and liver fibrosis.