Dysfunction of PDE4DIP contributes to LVNC development by regulating cell polarity, skeleton, and energy metabolism via Rho-ROCK pathway


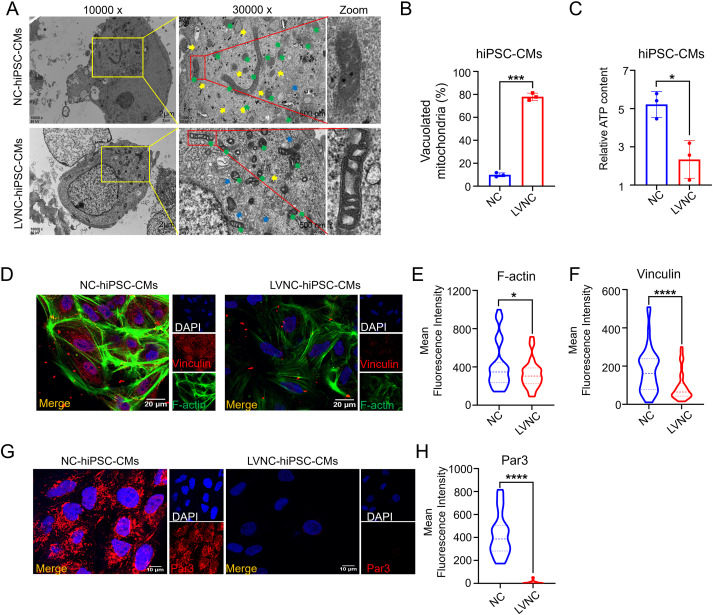
Left ventricular non-compaction (LVNC), is a hereditary cardiomyopathy with limited treatments. Our previous study linked phosphodiesterase 4D interacting protein (PDE4DIP) to LVNC development. To explore the functional role of PDE4DIP activation in regulating cell polarity, skeleton, and energy metabolism, and to elucidate its mechanisms driving LVNC development, bioinformatics analysis was performed to compare its expression in LVNC patients and normal subjects. Overexpression and knockdown of PDE4DIP were constructed in H9C2 cells and neonatal Sprague–Dawley rat primary cardiomyocytes, respectively. Electron microscopy, MitoTracker-Green staining, and an ATP kit were employed to assess mitochondria's morphology and functional status. Real-time quantitative PCR, western blotting, and immunofluorescence assays were employed to detect the expression of cell polarity-, skeleton-, and Rho-ROCK signaling-related genes and proteins. Cell scratching and CCK-8 assays were employed to detect cell migration and proliferation abilities of H9C2, respectively. We found that PDE4DIP expression was increased in the LVNC-derived human-induced pluripotent stem cell-derived cardiomyocytes compared with normal subjects. Furthermore, overexpression of PDE4DIP induced cytoskeletal disorganization, decreased ATP content and cell migration, and increased cell proliferation and mitochondrial vacuolation. Moreover, the knockdown of PDE4DIP promoted cytoskeleton formation and contributed to increased ATP content and elevated cell migration. Mechanically, overexpression of PDE4DIP inhibited cell polarity-, skeleton-, and Rho-ROCK signaling-related genes and proteins, which could be increased by knockdown of PDE4DIP, suggesting that a critical regulation of PDE4DIP to Rho-ROCK pathway. This discovery suggests that PDE4DIP contributes to the development of LVNC by regulating cell polarity, skeleton, and energy metabolism through the Rho-ROCK pathway.