Multi-omics analysis of epigenetic dysregulation reveals clinical heterogeneity and evaluates the immunotherapeutic potential of lung adenocarcinoma


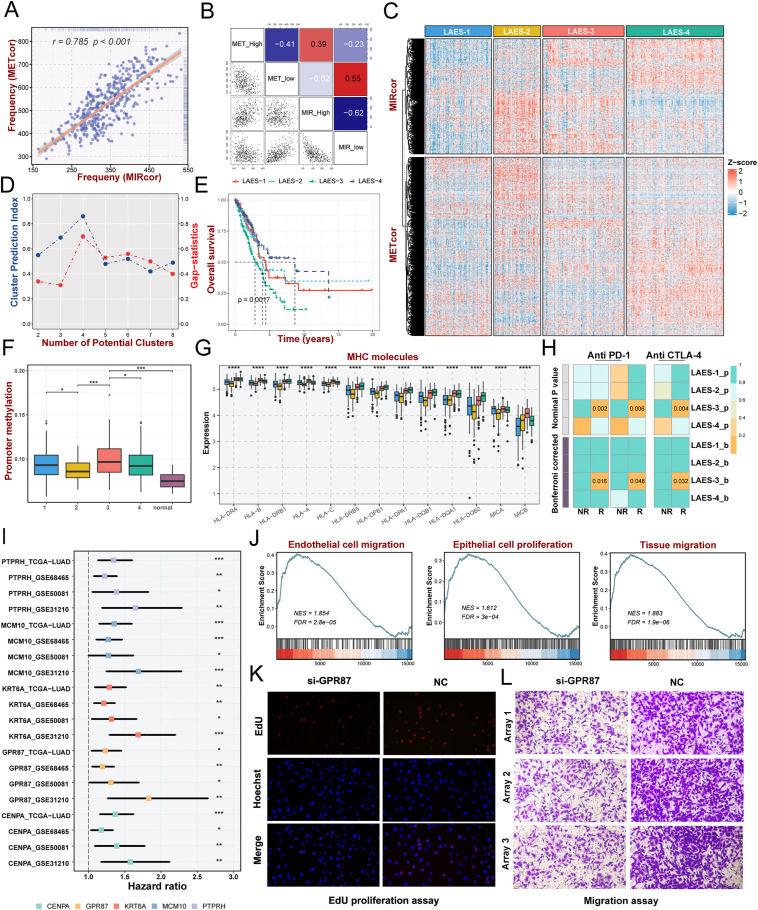
Lung cancer is the leading cause of cancer-related mortality globally, including small-cell lung cancer and non-small-cell lung cancer. As the most prevalent histological subtype of non-small-cell lung cancer, lung adenocarcinoma (LUAD) accounts for approximately 40% of all lung cancer cases.1 Due to the heterogeneity of LUAD, accurate categorization is required to create a treatment plan for LUAD patients, while the existing paradigm does not adequately capture the enormously heterogeneous characteristics of LUAD. The rise of epigenetics has brought new perspectives for tumor heterogeneity exploration. Epigenetic modifications, such as aberrant DNA methylation and microRNA (miRNA), are essential in controlling gene expression, heterogeneity, and clinical implication.2 Meanwhile, epigenetic disruptions contribute to lung cancer tumorigenesis, the generation of a malignant phenotype and aggression, and chemoresistance, which could serve as credible biomarkers for lung cancer molecular categorization, early diagnosis, prognosis classification, and treatment efficacy prediction.3 Through integrative clustering of the gene expression profiles regulated by epigenetics, we determined and validated four lung adenocarcinoma epigenetic subtypes (LAESs) with distinct prognoses and biological peculiarities from four independent multi-center lung adenocarcinoma cohorts.