Engrailed-1 inactivation leads to scarless skin wound healing through extracellular matrix remodeling


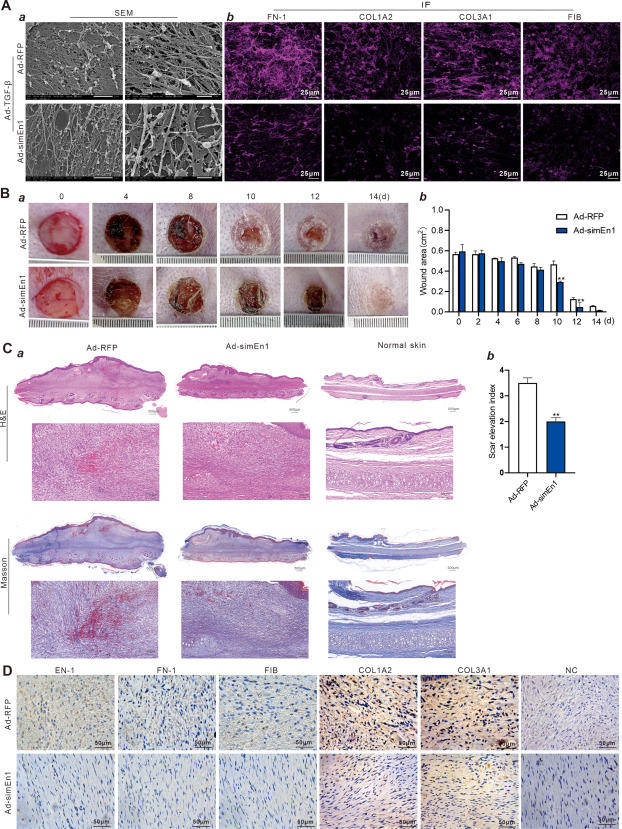
Hypertrophic scar and keloid are a major medical problem, which may lead to disfigurement, growth restriction, and permanent loss of function, causing severe physical, psychological, and economic burdens.1 When skin injury occurs, the wound heals through a dynamic series of physiological events, including blood clotting, granulation tissue formation, re-epithelialization, and extracellular matrix remodeling.2 However, the newly formed extracellular matrix in a scar may never achieve the flexibility or strength of the original tissue. Prior studies have suggested that the fibrotic process that occurs after skin injury may be mediated by a specific lineage of scar-prone fibroblasts in the dermis, which are responsible for scar deposition, namely engrailed-1 (EN-1) lineage-positive fibroblasts (EPFs).3 EN-1 is a transcription factor and plays an important role in embryonic development. In most cell types, EN-1 expression is limited to embryonic development. However, under pathological conditions, EN-1 can be re-expressed to promote phenotypic adaptation.4 The mechanical signaling factor YAP is associated with EPFs, establishing a link between mechanical transduction and fibrosis. Recent studies have demonstrated that EPFs play a key role in scar formation and that inhibition of YAP/EN-1 could restrict the formation of scar.5 However, as a downstream transcriptional factor of the YAP/TAZ pathway, EN-1's role in the pathological activation of fibroblasts and scar formation remains unclear. In this study, we investigated whether inhibition of EN-1 expression would be sufficient to suppress TGFβ1-induced fibroblast activation, extracellular matrix production, and scar formation in a skin injury model.