Autoantigenic peptide landscape of rheumatoid arthritis-associated HLA class II


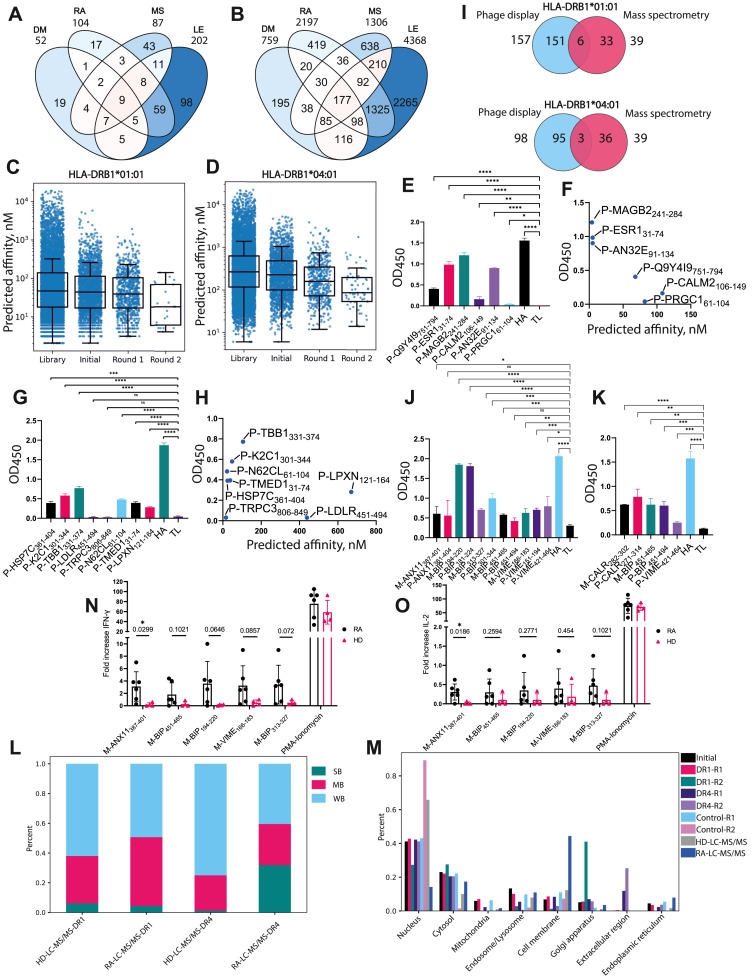
Rheumatoid arthritis (RA) is an autoimmune disorder characterized by synovial joint damage and progressive loss of mobility. The human leukocyte antigen (HLA) class II alleles HLA-DRB1∗01:01 and HLA-DRB1∗04:01 are strongly linked to RA susceptibility. Several autoantigenic peptides were reported to bind to RA-associated HLA-II and trigger autoreactive CD4+ T cell response. Here, we propose a dual combinatorial approach to identify novel autoantigenic peptides presented by HLA-II. We generated a phage library containing fragments of human autoantigens to screen for peptide ligands binding RA-associated HLA-II. Concurrently, the HLA-II immunopeptidome of peripheral blood mononuclear cells from RA patients was analyzed using liquid chromatography with tandem mass spectrometry (LC-MS/MS). This approach led to the identification of a panel of RA-associated HLA-II peptide ligands, confirmed via in vitro binding assay. Identified autoantigens include fragments of annexin A11, endoplasmic reticulum chaperone BiP, calreticulin, and vimentin. Finally, we demonstrated that the annexin A11 fragment, in the complex with HLA-DRB1∗01:01, can activate CD4+ T cells from RA patients.