Unraveling RUNX2 mutation in a cleidocranial dysplasia patient: Molecular insights into osteogenesis and proteostasis


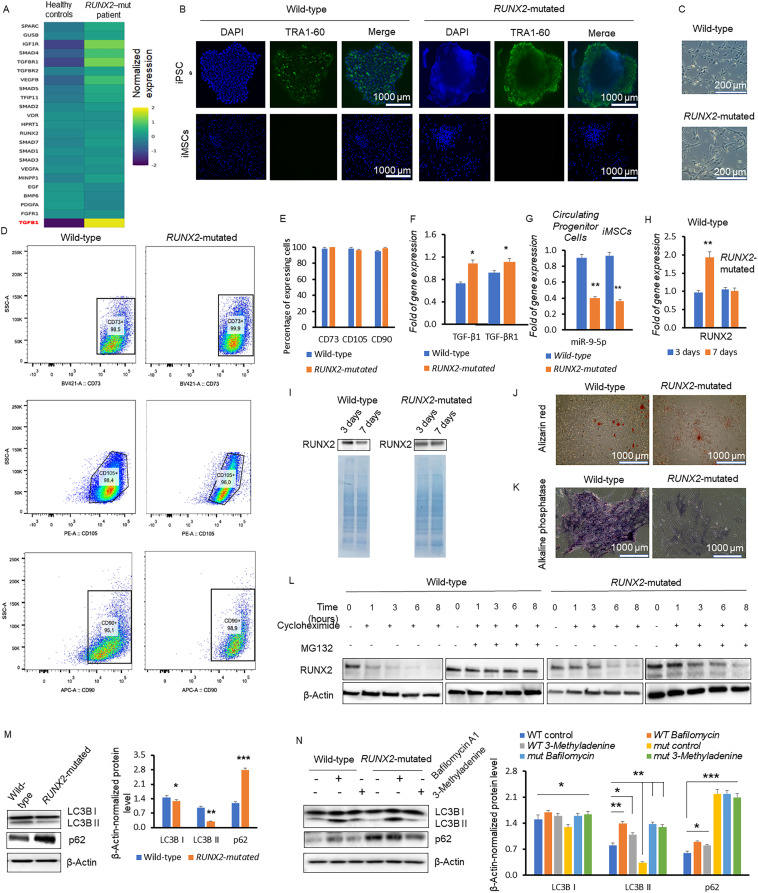
Runt-related transcription factor 2 (RUNX2), also called core-binding factor subunit alpha-1 (CBFA1), is the bone-specific transcription factor considered the master gene in osteogenesis, contains a crucial RUNT domain for DNA binding, and is regulated by multiple mechanisms. During the initial stages of osteogenesis, the expression levels of RUNX2 are primarily elevated and then gradually decrease during the formation of osteoblasts and osteocytes.1 Abnormal levels of RUNX2 can severely affect osteoblasts and skeletal structure. RUNX2 mutations are linked to cleidocranial dysplasia (CCD), a rare autosomal dominant skeletal disorder characterized by abnormal skeletal phenotypes.2 Our previous data demonstrate that RUNX2 mutations alter the modulation of p53 levels.2 However, despite the documented involvement of the RUNX2 protein in numerous cellular pathways beyond osteogenic differentiation, there are no in-depth studies on the impact of these mutations on cellular homeostasis. To explore the effects of mutations in the RUNT domain of RUNX2, here, we examined the cellular impact of the RUNX2 mutation c.505C > T in induced mesenchymal stem cells (iMSCs) obtained from induced pluripotent stem cells derived from one 26-year-old female CCD patient (Fig. S1). Our research provides the first molecular studies of this RUNX2 mutation in a CCD patient.