Preclinical ex vivo IL2RG gene therapy using autologous hematopoietic stem cells as an effective and safe treatment for X-linked severe combined immunodeficiency disease


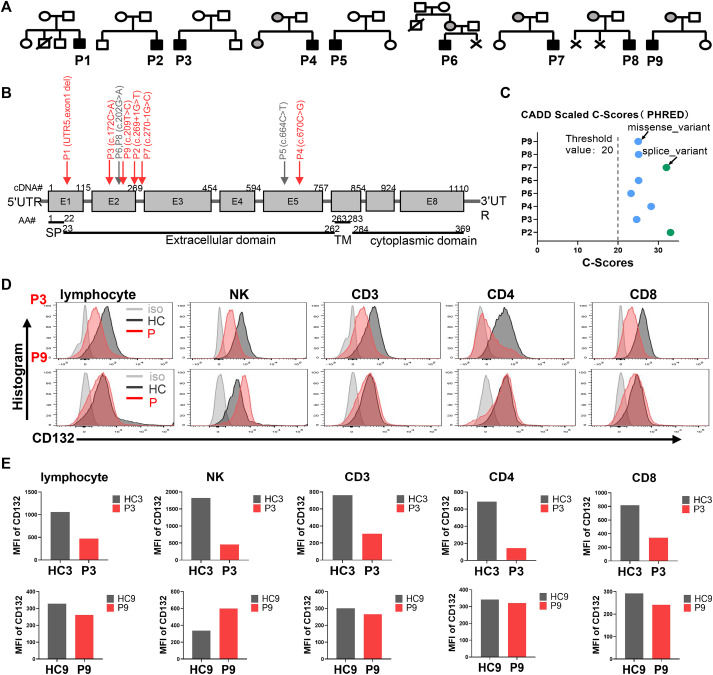
X-linked severe combined immunodeficiency disease (X-SCID) is a rare inherited disease caused by mutations in the interleukin 2 receptor subunit gamma gene (IL2RG), which encodes the common γ chain protein, a subunit of the receptor for lymphocytes. X-SCID is characterized by profound defects in T-cell, B-cell, and natural killer cell function. Here, we report a Chinese cohort of nine X-SCID patients with six novel IL2RG mutations. Among those, the two adolescent patients with an atypical immunotype were confirmed by further analyzing IL-2-JAK-STAT5 signaling, T cell proliferation, and T cell receptor excision circles (Trecs). Interestingly, Bacillus Calmette-Guérin (BCG) disease occurred commonly in this cohort. Although allogeneic hematopoietic stem-cell transplantation is curative for the disease, it is not available to all patients due to the lack of suitable matched donors. Autologous gene therapy using a self-inactivating lentiviral vector (SIN-LV) technology has provided an alternative therapy for such mono-genetic diseases. Here, we performed the pre-clinical studies to assess our SIN-LV carrying IL2RG on human ED7R cells deficient in IL2RG and CD34+ stem cells derived from the bone marrow of a healthy donor and a patient with X-SCID. This work is done complied with the established “Good Manufacturing Practice” (GMP) used in the clinical trials. In addition, a safety study is performed using the transduced CD34+ cells implanted into the axilla of nude mice in vivo. Overall, our studies have demonstrated the efficiency and safety of SIN-IL2RG-LV, which paves the way for conducting X-SCID gene therapy clinical trials in China in the near future.