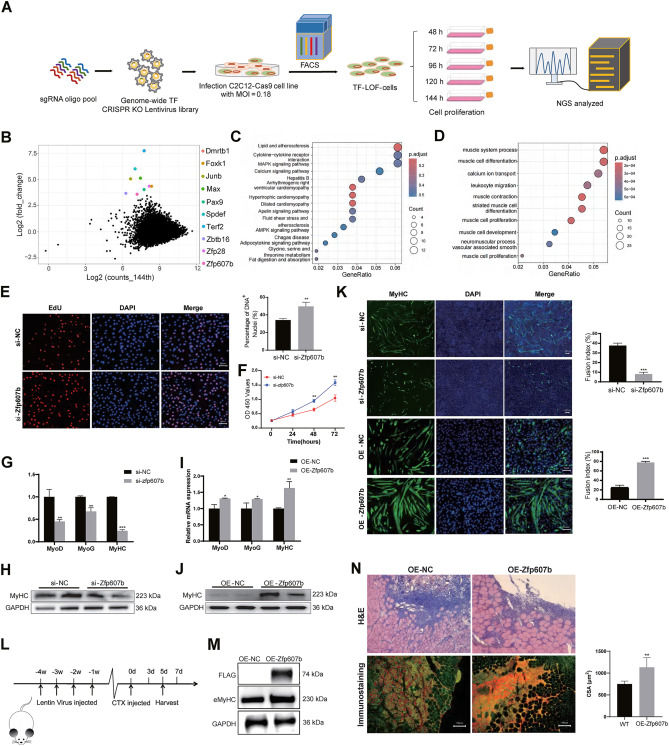
CRISPR/Cas9 screening reveals Zfp607b as a novel transcription factor regulating myogenesis


Skeletal muscle formation (myogenesis) is a complex process, and transcription factors (TFs) play an important role in controlling the phases of developmental myogenesis, particularly in myoblast proliferation and differentiation.1 However, the functions of numerous TFs in myoblast development and myogenesis remain unclear. To systematically identify TFs regulating myogenesis in skeletal muscle, we performed a genome-scale CRISPR-Cas9 loss-of-function (LOF) screen in mouse myoblast cells to identify TFs whose loss contributes to skeletal muscle proliferation. After screening, we identified 855 TFs closely associated with C2C12 cell proliferation. Functionally, we validated that Zfp607b improved skeletal muscle differentiation and regeneration using RNA interference knockdown in vitro and lentiviral injection in vivo. For the top fold-change genes in the TF knockout cell library, we explored potential mechanisms using transcriptomics and immunofluorescence. In conclusion, we constructed a genome-wide TF knockout cell library in myoblast and identified a novel TF, Zfp607b, that significantly participated in myogenesis and skeletal muscle regeneration. Our findings contribute to the understanding of the ZFP family involved in myogenesis and regeneration and provide a platform for studying the biological function of TFs in the future. Furthermore, our study offers valuable resources for understanding skeletal muscle development and human muscle-related diseases.