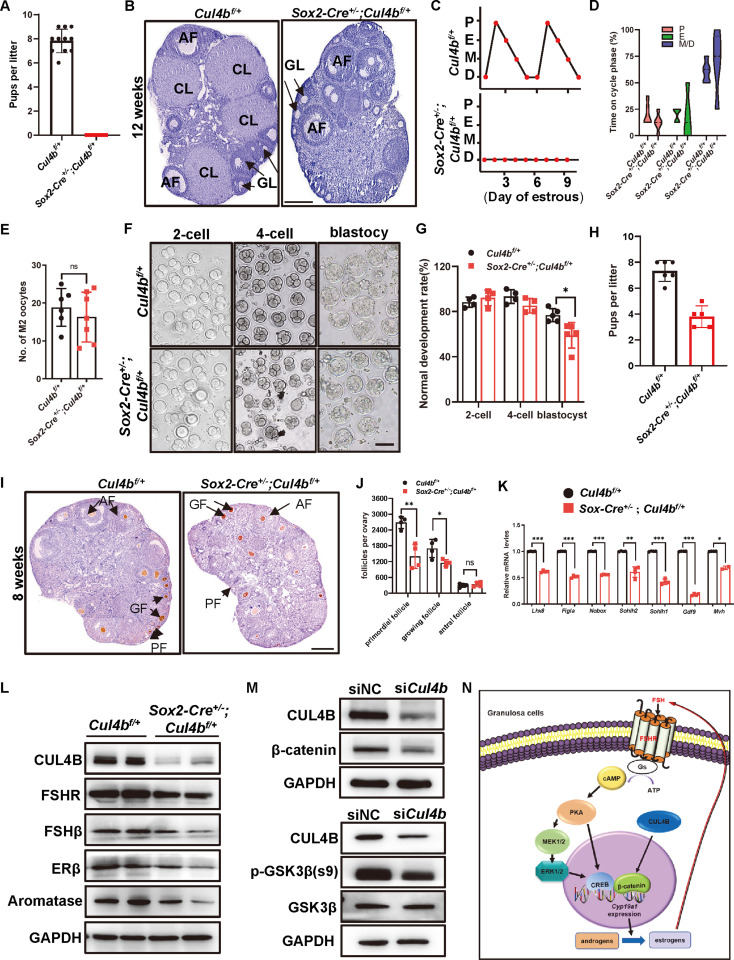
Heterozygous deletion of Cul4b in female mice leads to ovulatory dysfunction and female infertility


It is estimated that infertility impacts 8%–12% of reproductive-aged couples worldwide. Female infertility accounts for 37% of causes among infertile couples, and ovulatory dysfunction is regarded as its most common factor.1 CUL4B belongs to the Cullin family, whose members are the scaffolding proteins of Cullin-RING E3 ligases (CRLs). Human CUL4B gene mutations result in X-linked mental retardation syndromes. In addition to mental retardation, patients have symptoms such as short stature, obesity, and hypogonadism. Global (Sox2-Cre) or germ cell-specific (Vasa-Cre) Cul4b knockout male mice are infertile with impaired spermatozoa motility and spermatogonial stemness. DDB1 and DCAF1, two members of the CRL4A/B complex, can regulate oocyte survival, reprogramming,2 and meiotic maturation of oocytes.3 In this study, we generated Sox2-Cre+/−;Cul4bf/+ heterozygous female mice and found that these mice were infertile due to anovulation. CUL4B affects granulosa cell number and follicle development by regulating the follicle-stimulating hormone (FSH)/aromatase/estrogen loop. These results reveal a new function of CUL4B in follicular development and ovulation and provide a novel theoretical basis for the diagnosis and treatment of ovulation dysfunction and female infertility.