Aging-related inflammatory and metabolic disorder in the novel mutation of colony-stimulating factor-1 receptor (csf1r)P853T/+ in CSF1R-microglial encephalopathy


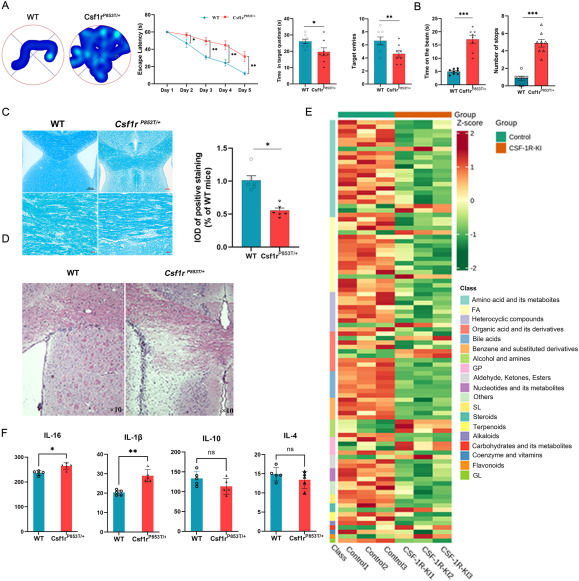
CSF1R-microglial encephalopathy (CME) is a rare dementia with rapid development of cognitive impairment. The main clinical features of CME are progressive cognitive impairment, motor dysfunction, and neuropsychiatric symptoms.1 Pathological features of CME showed diffuse leukoencephalopathy and thin corpus callosum, and immunohistochemical staining showed axonal bulbous transformation with pigmentary glial cells, extensive axonal degeneration, and myelin loss. The clinical phenotypes of CME are complex and varied, including progressive cognitive decline, movement disorders, seizures, psychobehavioral abnormalities, and multiple complications. The initial clinical manifestations of the disease vary greatly and are non-specific, so it is easy to be missed or misdiagnosed as Alzheimer's disease, multiple sclerosis, or other leukoencephalopathy. The average age of onset is 8–72 years (mean: 42 years) and the prognosis is poor with a median survival of 2–30 years (mean: 6 years). Current treatments include microglia replacement and symptomatic treatments, with no specific treatments for now. The main reason for this is that CME is difficult to diagnose because of its unknown mechanism, often requiring highly expensive gene sequencing.2 Most CSF1R mutation models reported to date are all knockout models, which cannot well mimic the clinical phenotype.3 CSF1R haploinsufficiency model mice show olfactory dysfunction, myelin hyperplasia, and increased reverse transcriptase of cortical oligodendrocytes, which have not been described in clinical lines.4 There has been no effective model of CME, and extensive knockout will cause gene deletions resulting in lethal defects. In this article, we proved that the CSF1RP853T/+ model can well imitate CME. This article explores the mechanisms of CME in the CSF1RP853T/+ mouse model and finds that CME suffers from abnormal intercellular communication, mitochondrial malformations, and enlarged lysosomes, worsening inflammation in the brain, which in turn exacerbate mitochondrial damage and form a vicious circle of predominantly immuno-inflammatory senescence. All animal protocols were approved by Yangzhou University's Institutional Animal Care and Use Committee and Animal Ethics Committee (No. YXYLL-2022-71), China, in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals.