Acyl-CoA thioesterase 7 is oncogenic in breast cancer by promoting oxidative phosphorylation via PGC1α


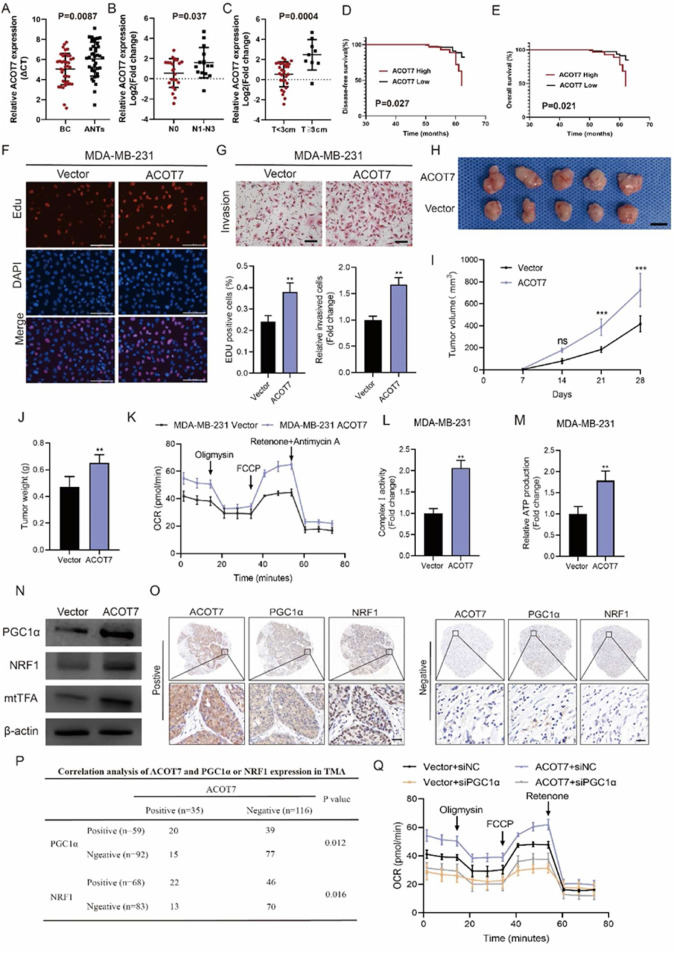
Metabolic reprogramming is a key feature of tumor cells and plays a key role in the adaptation of tumor cells to increased demands of biosynthesis and rapid proliferation.1 Numerous studies have shown that some key metabolic enzymes are essential for the initiation and progression of breast cancer (BC). These metabolic enzymes are involved in the regulation of many biological processes such as gene transcription, post-translational modification, and antioxidant capacity of cells, which endow tumor cells with the ability to adapt to divergent environmental stimuli.2 Therefore, it is of great significance to identify the role of important metabolic enzymes in the occurrence and development of BC to determine promising therapeutic targets. Free fatty acids are absorbed by cells and esterified with CoA to form acyl-CoA, which can be used as substrates for fatty acid oxidation or lipid synthesis. Acyl-CoA thioesterases (ACOTs) catalyze the hydrolysis of acyl-CoA to produce fatty acids and CoA in cells, thus maintaining the ratio of activated fatty acids to free fatty acids and the content of CoA in cells. ACOT7 exhibits broad specificity; it is active towards fatty acyl-CoAs with long chain lengths and has maximal activity toward arachidonic acid-CoA.3 It has been demonstrated that ACOT7 was the only member of ACOTs to be significantly up-regulated compared with non-tumoral BC tissues based on the GEPIA database. However, the precise role of ACOT7 in BC occurrence and development is still unknown. We found that the mRNA levels of ACOT7 in BC tissues were significantly higher than that in adjacent non-tumor tissues (Fig. 1A). ACOT7 mRNA expression was associated with more advanced clinicopathological parameters, including lymph node metastasis and tumor size (Fig. 1B, C). Kaplan–Meier plotter analysis indicated that a high level of ACOT7 mRNA was correlated with shorter distant metastasis-free survival and overall survival (Fig. S1A, B). Next, we used immunohistochemistry staining to examine the protein level of ACOT7 in human BC tissues in our cohort. Evaluation of ACOT7 expression levels was according to the staining of cytoplasmic ACOT7, and the score of intensity was also shown (Fig. S1C). Combined with the clinicopathological characteristics, we found that a high protein level of ACOT7 was correlated with advanced tumor size, lymph node metastasis, and Ki-67 index (Table S1). Importantly, survival analysis of our cohort indicated that high expression of ACOT7 protein in BC tissues was associated with reduced disease-free survival (P = 0.027) and overall survival (P = 0.021) (Fig. 1D, E). Next, we tested the protein level of ACOT7 in different BC cell lines (Fig. S1D). To explore the effects of ACOT7 on the proliferation and invasion of BC cells, we first established MDA-MB-231 and MCF-7 cells stably overexpressing ACOT7 by lentiviral infection (LV). Efficiency was verified by Western blot illustrated in Figure S2A. CCK-8 experiments indicated that ACOT7 overexpression increased cell viability in MDA-MB-231 and MCF-7 cells (Fig. S2B). EdU analysis demonstrated that ACOT7 overexpression enhanced the proliferative capabilities of MDA-MB-231 and MCF-7 cells (Fig. 1F; Fig. S2C). The transwell test demonstrated that ACOT7 overexpression remarkedly increased the invasive abilities of MDA-MB-231 and MCF-7 cells (Fig. 1G; Fig. S2D). To investigate the role of ACOT7 in tumor growth in vivo, MDA-MB-231 cells stably transfected with LV-ACOT7 RNA were subcutaneously implanted into BALB/c nude mice. One week later, tumor volumes were measured every seven days. On the 28th day, the mice were euthanized and tumor weights were measured (Fig. 1H). Compared with the control groups, ACOT7 overexpression in MDA-MB-231 cells significantly increased the volume and size of subcutaneous tumors in mice (Fig. 1I, J). Tumor xenografts of the LV-ACOT7 group displayed elevated expression of ACOT7 protein level and a significant increase in the abundance of Ki-67 positive cells (Fig. S2E, F). To further validate the effects of ACOT7 on the biological properties of BC cells, we then down-regulated ACOT7 expression in MDA-MB-231 and MCF-7 cells using RNA interference. Transfection efficiency was measured by Western blot analysis shown in Figure S3A. To investigate the role of ACOT7 in BC cell proliferation, we performed CCK8 and Edu experiments to measure changes in cell proliferation after the down-regulation of ACOT7 expression levels in MDA-MB-231 and MCF-7 cells. CCK-8 experiments indicated that ACOT7 knockdown decreased cell viability in MDA-MB-231 and MCF-7 cells (Fig. S3B). Similarly, EdU experiments demonstrated that ACOT7 knockdown impaired the proliferative ability of in MDA-MB-231 and MCF-7 cells (Figure S3C, D). The transwell test indicated that the invasion abilities of MDA-MB-231 and MCF-7 cells were significantly decreased after ACOT7 knockdown (Figure S3E, F).