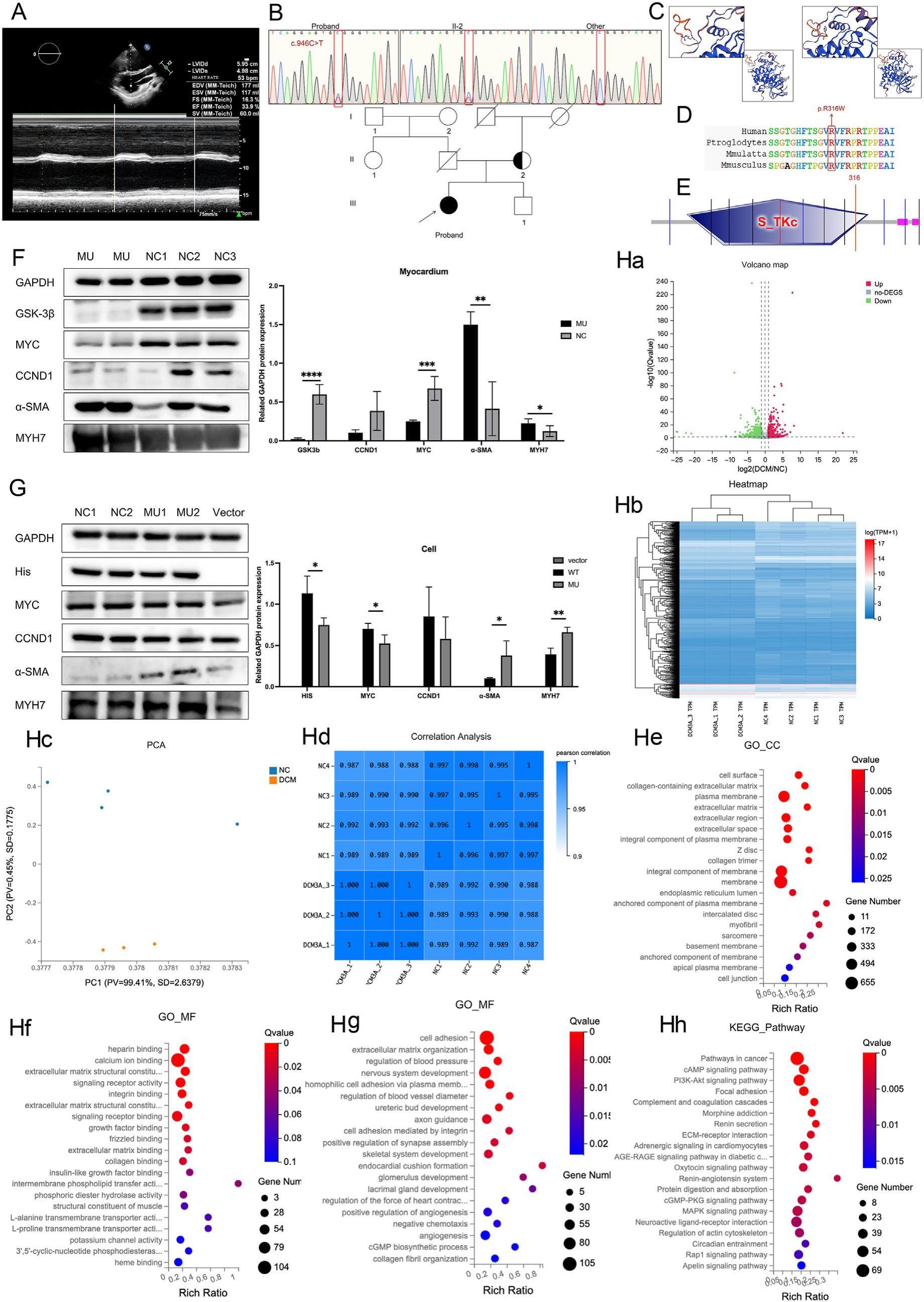
A novel mutation of glycogen synthase kinase-3β leads to a reduced level of GSK3β protein in a patient with dilated cardiomyopathy


Dilated cardiomyopathy (DCM) is a frequently inherited heart condition. A familial transmission rate of 20%-35% has been documented, and most of these cases are autosomal dominant. This condition has a high rate of morbidity and mortality, which can lead to cardiac arrest and persistent heart failure. Several biological processes are regulated by the constitutively active serine/threonine protein kinase glycogen synthase kinase-3β (GSK-3β), constitutively active. It is a negative regulator of glucose homeostasis and is involved in energy metabolism, inflammation, endoplasmic reticulum stress, mitochondrial dysfunction, and apoptotic pathways. E-cadherin is repressed in epithelialemesenchymal transition (EMT), allowing the passage of attached epithelial cells to the mesenchymal state. B-Catenin represses E-cadherin expression to promote EMT. In the WNT pathway, GSK-3β regulates E-cadherin transcription by phosphorylating bcatenin to trigger proteasomal degradation. Although numerous mechanisms remain unknown, GSK-3β has demonstrated a significant role in heart cardiomyocyte differentiation, hypertrophy, and fibrosis. The ability of GSK-3β to control cardiac hypertrophy, which can both cause and treat disease, may have a dosage-dependent impact. Abnormalities in the cardiac Z-disc were found in GSK-3β conditional knockout mice, which affected myocardial contraction. Few people with primary GSK-3β mutations have been identified, and prior research has mostly focused on gene-edited mice or cells. A potentially pathogenic GSK-3β variant was identified in the current study in a patient with DCM, which has significant ramifications for future genetic research on both DCM and GSK-3β.